pH and Taste
- Page ID
- 50888
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)While the ability to calculate the pH of a solution from the hydronium-ion concentration and vice versa is useful, it is not the only thing we need to understand about pH. If someone gives you a solution whose pH is 14.74, it is true that the hydronium-ion concentration must be
1.82 × 10–15 mol dm–3 but it is perhaps more important to know that the solution is corrosively basic and should be handled with respect. In general, then, we need not only to be able to calculate a pH but also to have some realization of what kind of solutions have what kind of pH. The following table is designed to meet this need. It is also part of our collection of acid-base resources.
Table \(\PageIndex{1}\) The pH Scale.
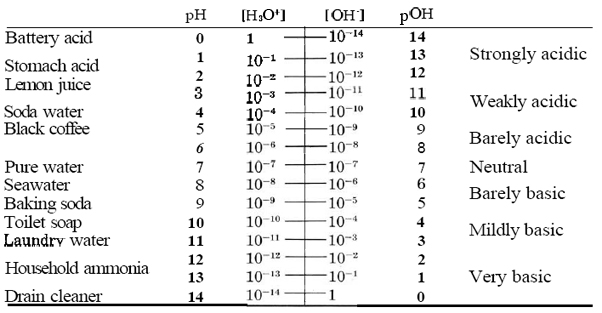
In pure water at 25°C the hydronium-ion concentration is close to 1.00 × 10–7 mol dm–3, so that the pH is 7. In consequence any solution, not only pure water, which has a pH of 7 is described as being neutral. An acidic solution, as we know, is one in which the hydronium-ion concentration is greater than that of pure water, i.e., greater than 10–7 mol dm–3. In pH terms this translates into a pH which is less than 7 (because the pH is a negative logarithm). Small pH values are thus characteristic of acidic solutions; the smaller the pH, the more acidic the solution.
By contrast, a basic solution is one in which the hydroxide-ion concentration is greater than 10–7 mol dm–3. In such a solution the hydronium-ion concentration is less than 10–7 mol dm–3, so that the pH of a basic solution is greater than 7. Large pH values are thus characteristic of basic solutions. The larger the pH, the more basic the solution.
We have some direct experience of pH through our sense of taste, which responds to the concentration of hydronium ions. Most of us are able to detect a sour, tart, acidic taste in a solution with a pH of between 4 and 5. Black coffee with a pH of 5 does not taste acidic to most people, whereas carbonated water (soda water) with a pH of 4 does. A somewhat more acidic solution with a pH of 3 or even 2 tastes pleasantly acidic, especially if sweetened with sugar. Most fruit juices and most soft drinks have a pH in this range. If the pH of the solution is lower than 2, the taste is too tart for us to tolerate it for long. When someone experiences 'acid reflux', they experience the taste of a solution with a pH of 1.4, since this is the normal pH of stomach acid. Any pH below this value is not only unpleasant to the taste but acidic enough to attack the skin. Note that a sufficiently concentrated solution of strong acid can actually have a negative pH. Sulfuric acid in a car battery has a concentration of about 5 mol H2SO4 dm–3 and a pH of about – 0.7.
Since we do not often intentionally swallow basic solutions, we cannot rely on the sense of taste to guide us through pH values which are greater than 7. Examples of very weakly basic solutions are sodium bicarbonate (baking soda, NaHCO3), which has a pH close to 8, and a solution of soap, which usually has a pH slightly greater than 9. Solutions with a pH of 10 or 11 (which we can describe as mildly basic) are to be found in the weekly wash. Most laundry powders contain a weak base such as a phosphate or a carbonate, which raises the pH to this value. Solutions with a pH of 11 feel noticeably “soapy” to the touch and also cause a characteristic wrinkling of the skin. If the pH is greater than 12 or 13, the solution attacks skin rapidly enough to be dangerous. Very basic solutions are more corrosive to skin than very acidic solutions. A solution of drain cleaner (mainly with scent and coloring matter added) has a pH of between 14 and 15 and should be handled only with the utmost caution.
From ChemPRIME: 14.2: pH and pOH
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


