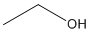7.3: Ionic Compounds - Names and Formulas
- Page ID
- 465543
Chemistry is the experimental and theoretical study of materials on their properties at the macroscopic and microscopic levels.
A chemical formula is a format used to express the structure of atoms. The formula tells which elements and how many of each element are present in a compound. Formulas are written using the elemental symbol of each atom and a subscript to denote the number of elements.
Molecular Formula
The molecular formula is based on the actual makeup of the compound. Although the molecular formula can sometimes be the same as the empirical formula, molecular compounds tend to be more helpful. However, they do not describe how the atoms are put together.
Ex. Molecular Formula for Ethanol: C2H6O.
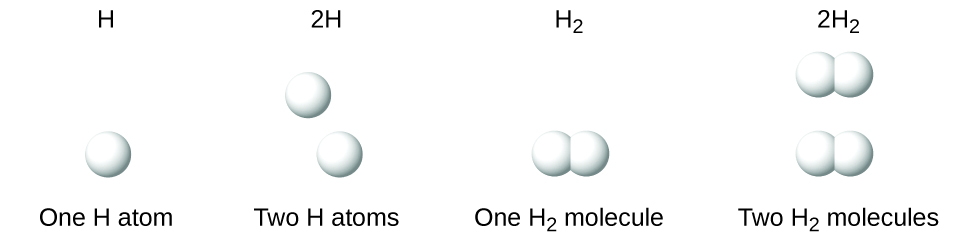
It is important to note that a subscript following a symbol and a number in front of a symbol does not represent the same thing; for example, H2 and 2H represent distinctly different species. H2 is a molecular formula; it represents a diatomic molecule of hydrogen, consisting of two atoms of the element that are chemically bonded together. The expression 2H, on the other hand, indicates two separate hydrogen atoms that are not combined as a unit. The expression 2H2 represents two molecules of diatomic hydrogen (Figure \(\PageIndex{1}\)).
Empirical Formula
An empirical formula shows the most basic form of a compound. Empirical formulas show the number of atoms of each element in a compound in the most simplified state using whole numbers. While empirical formulas cannot determine the structure, shape, or properties of the compound, it usually is one of the first experiments to know what atoms and in which relationship are present in an unknown sample.
Ex. Find the empirical formula for C8H16O2.
Answer: C4H8O (divide all subscripts by 2 to get the smallest, whole number ratio).
Binary compounds
Binary compounds are formed between two elements, either a metal paired with a nonmetal or two nonmetals paired together.
When two nonmetals are paired together, the compound is a molecular compound always represented using the Molecular Formula. When writing out the formula, the element with a more metallic character (with a positive oxidation state) is placed first.
Ex. Molecular Compound: N2O4 (Dinitrogen Tetroxide)
When a metal is paired with a nonmetal, they form an ionic compound, representing it using Empirical Formulas. In ionic compounds, each atom is in the form of an ion; one atom is a negatively charged ion (anion), and the other is a positively charged ion (cation). The net charge of the compound must be neutral using the minimal amount of each atom.
Ex. Ionic Compound: BaBr2 (Barium Bromide)
: Empirical and Molecular Formulas
Molecules of glucose (blood sugar) contain 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. What are the molecular and empirical formulas of glucose?
Solution
The molecular formula is C6H12O6 because one molecule contains 6 C, 12 H, and 6 O atoms. The simplest whole-number ratio of C to H to O atoms in glucose is 1:2:1, so the empirical formula is CH2O.
A molecule of metaldehyde (a pesticide used for snails and slugs) contains 8 carbon atoms, 16 hydrogen atoms, and 4 oxygen atoms. What are the molecular and empirical formulas of metaldehyde?
- Answer
-
Molecular formula, C8H16O4; empirical formula, C2H4O
-
Structural Formula
A structural formula displays the atoms of the molecule in the order they are bonded. It also depicts how the atoms are bonded to one another, for example single, double, and triple covalent bond. Covalent bonds are shown using lines. The number of dashes indicates whether the bond is a single, double, or triple covalent bond. Structural formulas are helpful because they explain the compound's properties and structure, which empirical and molecular formulas cannot always represent.
Condensed Structural Formula
Condensed structural formulas show the order of atoms like a structural formula but are written in a single line to save space and make it more convenient and faster to write out. Condensed structural formulas are also helpful when showing that a group of atoms is connected to a single atom in a compound. When this happens, parenthesis is used around the group of atoms to show they are together.
Ex. Condensed Structural Formula for Ethanol: CH3CH2OH (Molecular Formula for Ethanol C2H6O).
Line-Angle Formula
Because organic compounds can sometimes be complex, line-angle formulas are used to write carbon and hydrogen atoms more efficiently by replacing the letters with lines. A carbon atom is present wherever a line intersects another line. Hydrogen atoms are then assumed to complete each of carbon's four bonds. All other atoms that are connected to carbon atoms are written out. Line angle formulas help show the structure and order of the atoms in a compound, making the advantages and disadvantages similar to structural formulas.
Ball-and-stick model
A ball-and-stick model shows the geometric arrangement of the atoms with atomic sizes not to scale, and a space-filling model shows the relative sizes of the atoms.
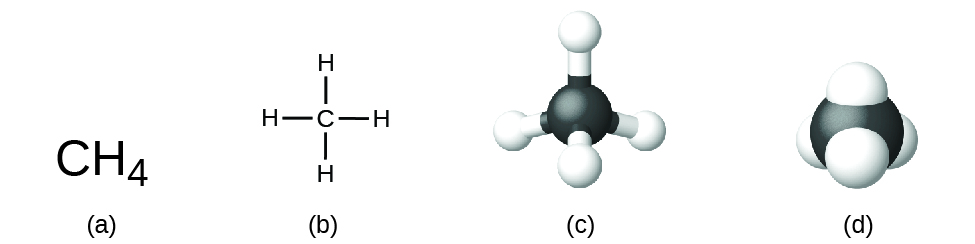
Molecular Geometry and Structural Formula
Understanding how atoms in a molecule are arranged and how they are bonded together is very important in giving the molecule its identity. Isomers are compounds in which two molecules can have the same number of atoms, and thus the same molecular formula, but can have completely different physical and chemical properties because of differences in the structural formula.
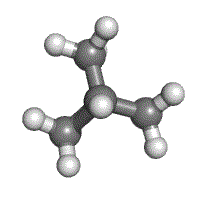
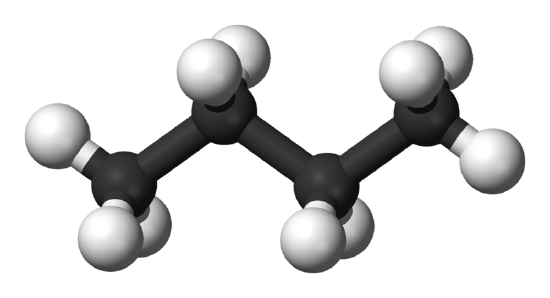
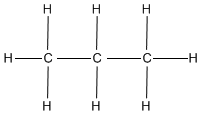
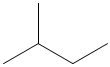
Methylpropane and butane have the same molecular formula of C4H10, but are structurally different (methylpropane on the left, butane on the right).
Summary
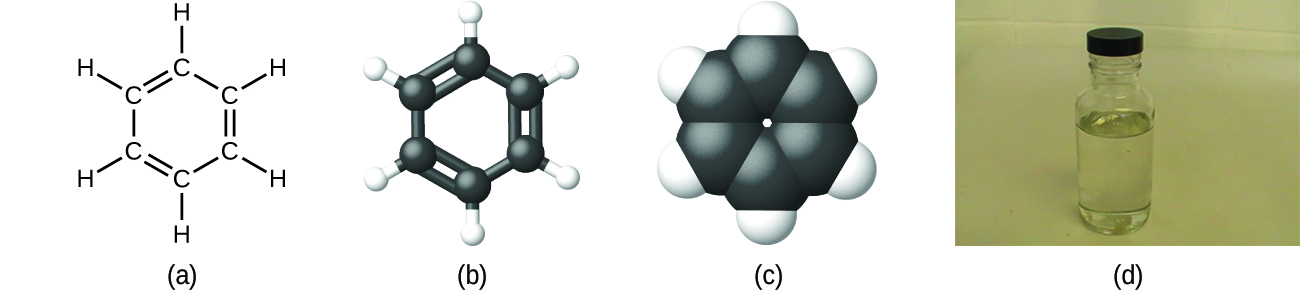
As discussed previously, we can describe a compound with a molecular formula, in which the subscripts indicate the actual numbers of atoms of each element in a molecule of the compound. In many cases, the molecular formula of a substance is derived from the experimental determination of both its empirical formula and its molecular mass (the sum of atomic masses for all atoms composing the molecule). For example, it can be determined experimentally that benzene contains two elements, carbon (C) and hydrogen (H), and that for every carbon atom in benzene, there is one hydrogen atom. Thus, the empirical formula is CH. An experimental determination of the molecular mass reveals that a molecule of benzene contains six carbon atoms and six hydrogen atoms, so the molecular formula for benzene is C6H6 (Figure \(\PageIndex{3}\)).
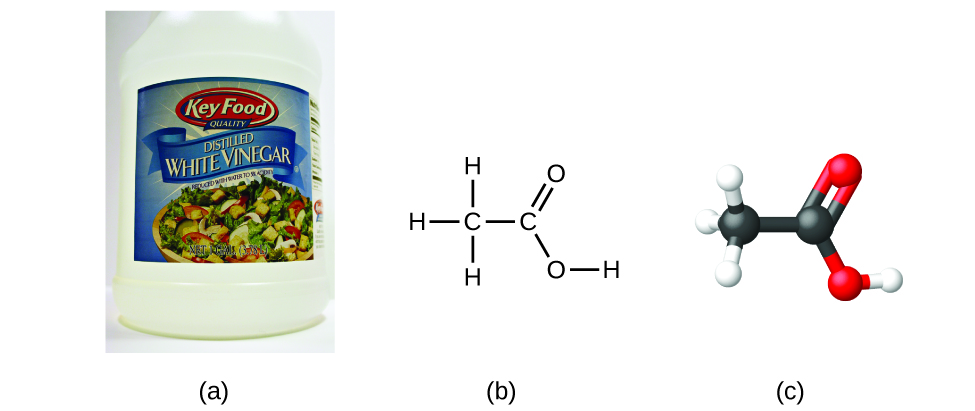
If we know a compound’s molecular formula, we can easily determine the empirical formula. (This is somewhat of an academic exercise; the reverse chronology is generally followed in actual practice.) For example, the molecular formula for acetic acid, the component that gives vinegar its sharp taste, is C2H4O2. This formula indicates that a molecule of acetic acid (Figure \(\PageIndex{4}\)) contains two carbon atoms, four hydrogen atoms, and two oxygen atoms. The ratio of atoms is 2:4:2. Dividing by the lowest common denominator (2) gives the simplest, whole-number ratio of atoms, 1:2:1, so the empirical formula is CH2O. Note that a molecular formula is always a whole-number multiple of an empirical formula.
- Which of the following formulas can not be considered an Empirical Formula?
- HClO
- C5H10
- CO2
- Write the empirical formula for the following compounds
- C12H10O6
- CH3CH2CH2CH2CH2CH2CH3
- H3O+
- Answer
-
- b. C5H10
- a. C6H5O3, b. C7H16, c. H3O+
Contributors and Attributions
- Sol Parajon Puenzo (Cañada College)
- Jean Kim (UCD), Kristina Bonnett (UCD)