4.1: Characteristics of Covalent Compounds
- Page ID
- 341902
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Recognize bond characteristics of covalent compounds: bond length and bond energy
Bond Length and Bond Energy
Bond length is the separation distance between two nuclei forming a covalent bond. The bond length is usually measured in meters or picometers (1 pm = 1x10-12m). For example, the covalent bond in the hydrogen molecule (H2) has a certain length (about 7.4 × 10−11 m). Other covalent bonds also have known bond lengths, which are dependent on both the identities of the atoms in the bond and whether the bonds are single, double, or triple bonds. Table \(\PageIndex{1}\) lists the approximate bond lengths for some single covalent bonds. The exact bond length may vary depending on the identity of the molecule but will be close to the value given in the table.
Bond energy is defined as the energy required to break a particular bond in a molecule in the gas phase, expressed in kilojoules per mole of bonds (kJ/mol). This value depends on the identity of the bonded atoms and the nature of the molecule (for example, the energy required to break the O-H bond in H2O is different than in H2O2). Except for diatomic molecules, the bond energies listed in Table \(\PageIndex{1}\) are average values for all bonds of a given type in a range of molecules.
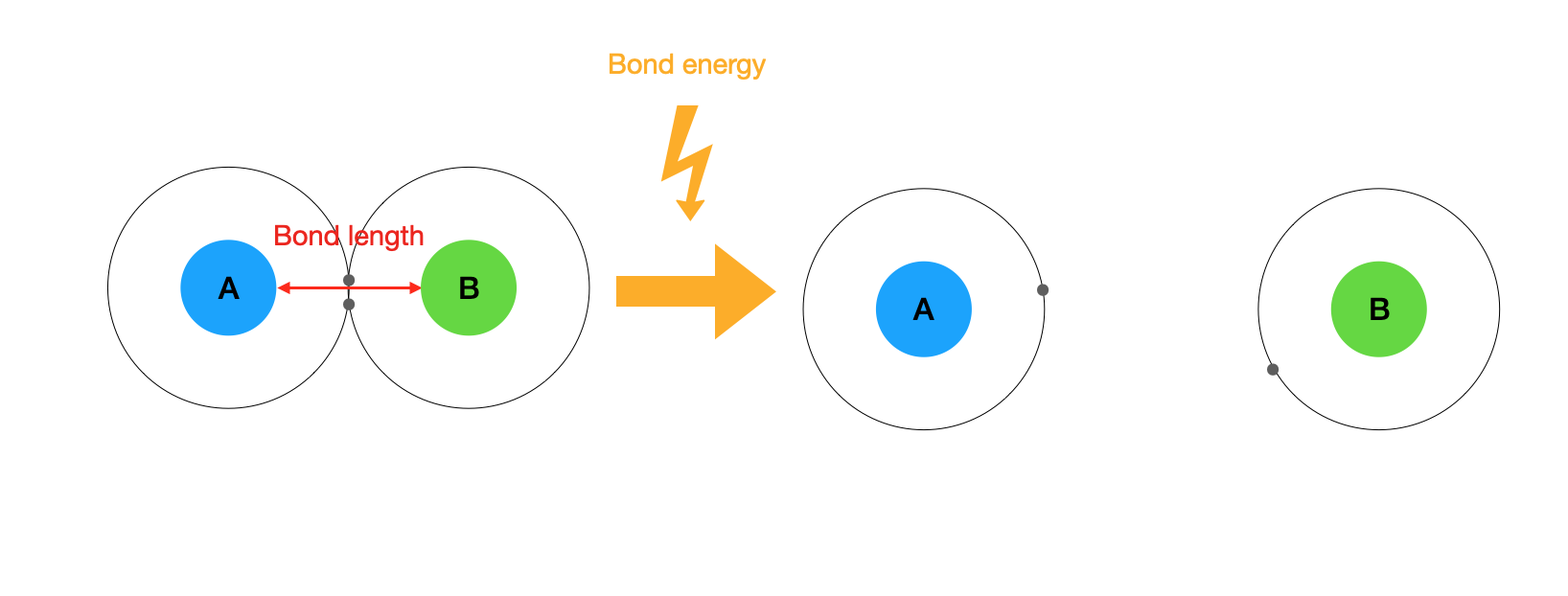
Figure \(\PageIndex{1}\): representation of bond length and bond energy between two atoms A and B
| Bond | Length (× \(10^{−12}\, m\)) | Bond Energy (kJ/mol) |
|---|---|---|
| H–H | 74 | 436 |
| H–C | 110 | 414 |
| H–N | 100 | 386 |
| H–O | 97 | 459 |
| H–I | 161 | 295 |
| C–C | 154 | 350 |
| C–N | 147 | 305 |
| C–O | 143 | 358 |
| N–N | 145 | 167 |
| O–O | 145 | 142 |
Table \(\PageIndex{2}\) compares the lengths and bond energies of single covalent bonds with those of double and triple bonds between the same atoms. Without exception, as the number of covalent bonds between two atoms increases, the bond length decreases and the bond energy increases.
By comparing both tables, we can conclude that the more energy needed to break a bond, the higher its stability, and the shorter the bond length between the atoms
| Bond | Length (× \(10^{−12}\, m\)) | Bond Energy (kJ/mol) |
|---|---|---|
| C–C | 154 | 350 |
| C=C | 134 | 614 |
| C≡C | 120 | 839 |
| C–N | 147 | 305 |
| C=N | 128 | 615 |
| C≡N | 116 | 891 |
| C–O | 143 | 358 |
| C=O | 120 | 745 |
| C≡O | 113 | 1072 |
| N–N | 145 | 167 |
| N=N | 123 | 918 |
| N≡N | 110 | 941 |
| O–O | 145 | 142 |
| O=O | 121 | 495 |
Physical properties of covalent compounds
Covalent compounds form discrete molecules that can exist independently from each other, in contrast to the crystal structure of ionic compounds in which formula units cannot exist individually but only as a part of a lattice of ions. Therefore, the physical properties of covalent molecules depend heavily on the nature of their interaction with other molecules (intermolecular forces). Depending on the nature of these intermolecular interactions, covalent compounds may exist as a solid, a liquid, or a gas at room temperature and normal atmospheric pressure. As a general rule, we can say that covalent compounds have lower melting and boiling points compared to ionic compounds with similar molecular mass.
Since there are no ions in their structure, covalent compounds do not usually exhibit any electrical conductivity, either in pure form or when dissolved in water. These exceptions involve ionizable covalent compounds such as acids (these molecules dissociate in water forming ions, and therefore, solutions of ionizable covalent compounds can conduct the electricity), or conjugated.

Pi bonds form an extended area of overlapping atomic orbitals sharing electrons. The electrons are delocalized throughout them, no longer shared between just two atoms.
Key Takeaways
- Covalent bonds between different atoms have different bond lengths and bond energies.
- The stronger the covalent bond, the higher its bond energy and the shorter its bond distance
- As a general rule, covalent compounds have lower melting and boiling points compared to ionic compounds with similar molecular mass.
- The physical properties of covalent molecules depend heavily on the nature of their interaction with other molecules
Citations and Attributions
- Characteristics of Covalent Bonds. (2021, March 21). Retrieved May 19, 2021, from https://chem.libretexts.org/@go/page/16131
- Bond Energies. (2020, August 21). Retrieved May 19, 2021, from https://chem.libretexts.org/@go/page/1981

