6.E: Covalent Bonding and Simple Molecular Compounds (Exercises)
- Last updated
- Save as PDF
- Page ID
- 402467

- Anonymous
- LibreTexts
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
4.1: Covalent Bonds
Concept Review Exercises
- How is a covalent bond formed between two atoms?
- How does covalent bonding allow atoms in group 6A to satisfy the octet rule?
Answers
- Covalent bonds are formed by two atoms sharing electrons.
- The atoms in group 6A make two covalent bonds.
Exercises
- Define covalent bond.
- What is electron sharing?
- Draw the Lewis diagram for the covalent bond in the H2 molecule.
- Draw the Lewis diagram for the covalent bond in the Br2 molecule.
- Draw the Lewis diagram for the covalent bond in the HCl molecule.
- What is the difference between a molecule and a formula unit?
- Why do hydrogen atoms not follow the octet rule when they form covalent bonds?
- Draw the Lewis diagram for the covalent bonding in H2S. How many bonding electrons and nonbonding electrons are in the molecule?
- Draw the Lewis diagram for the covalent bonding in CF4. How many bonding electrons and nonbonding electrons are in the molecule?
- Draw the Lewis diagram for the covalent bonding in PCl3. How many bonding electrons and nonbonding electrons are in the molecule?
- How many covalent bonds does a hydrogen atom typically form? Why?
- How many covalent bonds does an oxygen atom typically form? Why?
- Tellurium atoms make covalent bonds. How many covalent bonds would a tellurium atom make? Predict the formula of a compound between tellurium and hydrogen.
- Tin atoms make covalent bonds. How many covalent bonds would a tin atom make? Predict the formula of a compound between tin and hydrogen.
- Astatine is a synthetic element, made one atom at a time in huge “atom-smasher” machines. It is in the halogen group on the periodic table. How many covalent bonds would an atom of this element form?
- There have been reports that atoms of element 116 (Lv) were made by smashing smaller atoms together. Using the periodic table, determine what column element 116 would be in and suggest how many covalent bonds an atom of this element would form.
Answers
1. A covalent bond is formed when two atoms share electrons.
2. Electron sharing joins two atoms in a covalent bond. This is a more stable arrangement than 2 individual atoms.
3. 

4. 
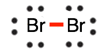
5. 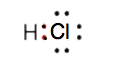

6. A molecule is a discrete combination of atoms; a formula unit is the lowest ratio of ions in a crystal. 7. Hydrogen atoms follow the duet rule (not the octet rule). This is because it has only one shell and this shell can only hold 2 electrons.
8.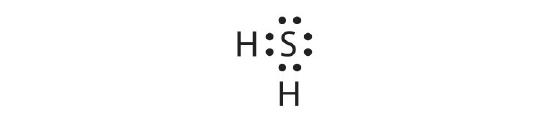 bonding electrons: 4; nonbonding electrons: 4
9.
bonding electrons: 4; nonbonding electrons: 4
9.
 bonding electrons: 8; nonbonding electrons: 24
bonding electrons: 8; nonbonding electrons: 24
10.

bonding electrons: 6; nonbonding electrons: 20
11. Hydrogen atoms form only one covalent bond because they have only one valence electron to pair.
12. Oxygen atoms form 2 covalent bonds because oxygen atoms have 6 valence electrons (2 lone pairs plus 2 unpaired electrons that are shared to achieve octet).
13. two; H2Te
14. four: SnH4
15. one
16. two
4.2: Covalent Compounds - Formulas and Names
Concept Review Exercises
- How do you recognize a covalent compound?
- What are the rules for writing the molecular formula of a simple covalent compound?
- What are the rules for naming a simple covalent compound?
Answers
- A covalent compound is usually composed of two or more nonmetal elements.
- It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name.
- Name the first element first and then the second element by using the stem of the element name plus the suffix -ide. Use numerical prefixes if there is more than one atom of the first element; always use numerical prefixes for the number of atoms of the second element.
Exercises
- Identify whether each compound has covalent bonds.
- NaI
- Na2CO3
- N2O
- SiO2
- Identify whether each compound has covalent bonds.
- C2H6
- C6H5Cl
- KC2H3O2
- Ca(OH)2
- Identify whether each compound has ionic bonds, covalent bonds, or both.
- Na3PO4
- K2O
- COCl2
- CoCl2
- Identify whether each compound has ionic bonds, covalent bonds, or both.
- FeCl3
- Fe(NO3)3
- (NH2)2CO
- SO3
- Which is the correct molecular formula—H4Si or SiH4? Explain.
- Which is the correct molecular formula—SF6 or F6S? Explain.
- Write the name for each covalent compound.
- SiF4
- NO2
- CS2
- P2O5
- Write the name for each covalent compound.
- CO
- S2O3
- BF3
- GeS2
- Write the formula for each covalent compound.
- iodine trichloride
- disulfur dibromide
- arsenic trioxide
- xenon hexafluoride
- Write the formula for each covalent compound.
- boron trichloride
- carbon dioxide
- tetraphosphorus decoxide
- germanium dichloride
- Write two covalent compounds that have common rather than systematic names.
- What is the name of the simplest organic compound? What would its name be if it followed the nomenclature for binary covalent compounds?
Answers
-
- no
- yes
- yes
- yes
2.
- yes
- yes
- yes
- yes
-
- both
- ionic
- covalent
- ionic
4.
- ionic
- both
- covalent
- covalent
- SiH4; except for water, hydrogen is almost never listed first in a covalent compound.
6. SF6; the less electronegative atom (S) is written first
-
- silicon tetrafluoride
- nitrogen dioxide
- carbon disulfide
- diphosphorus pentoxide
8.
- carbon monoxide
- disulfur trioxide
- boron trifluoride
- germanium disulfide
-
- ICl3
- S2Br2
- AsO3
- XeF6
10.
- BCl3
- CO2
- P4O10
- GeCl2
- H2O and NH3 (water and ammonia) (answers will vary)
- CH4; carbon tetrahydride
4.3: Drawing Lewis Structures
Exercises
- What is one clue that a molecule has a multiple bond?
2. Draw the Lewis diagram for each of the following.
a. H2O
b. NH3
c. C2H6
d. CCl4
3. Each molecule contains double bonds. Draw the Lewis diagram for each. The first element is the central atom.
- CS2
- C2F4
- COCl2
4. Each molecule contains multiple bonds. Draw the Lewis diagram for each. Assume that the first element is the central atom, unless otherwise noted.
- N2
- CO
- HCN (The carbon atom is the central atom.)
- POCl (The phosphorus atom is the central atom.)
5. Explain why hydrogen atoms do not form double bonds.
6. Why is it incorrect to draw a double bond in the Lewis diagram for MgO?
Answers
- If single bonds between all atoms do not give all atoms (except hydrogen) an octet, multiple covalent bonds may be present.
- a.
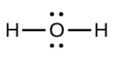
b.
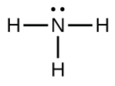
c.
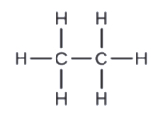
d.
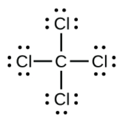
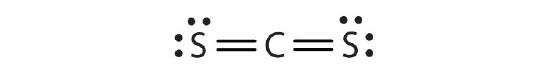 b.
b.
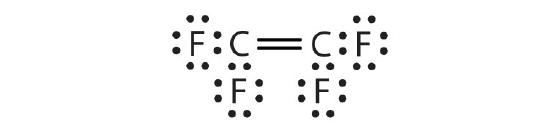 c.
c.
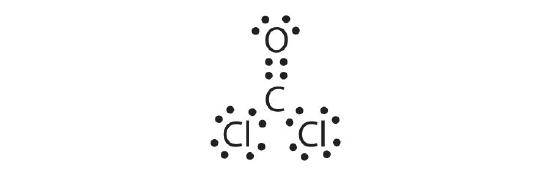
4. a. 
b. 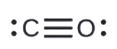
c. 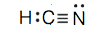
d.
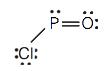
5. Hydrogen can accept only one more electron; multiple bonds require more than one electron pair to be shared.
6. MgO is an ionic compound (Mg transfers two electrons to O). The electrons are not shared hence it's incorrect to draw a double bond.
This is the Lewis dot structure of MgO.
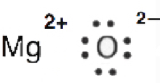
4.4: Characteristics of Covalent Bonds
Concept Review Exercises
- What is the name for the distance between two atoms in a covalent bond?
- What does the electronegativity of an atom indicate?
- What type of bond is formed between two atoms if the difference in electronegativities is small? Medium? Large?
Answers
- bond length
- Electronegativity is a qualitative measure of how much an atom attracts electrons in a covalent bond.
- nonpolar; polar; ionic
Exercises
- Which is longer—a C–H bond or a C–O bond? (Refer to Table \(\PageIndex{1}\).)
- Which is shorter—an N–H bond or a C–H bond? (Refer to Table \(\PageIndex{1}\).)
- A nanometer is 10−9 m. Using the data in Table \(\PageIndex{1}\) and Table \(\PageIndex{2}\), determine the length of each bond in nanometers.
- a C–O bond
- a C=O bond
- an H–N bond
- a C≡N bond
- An angstrom (Å) is defined as 10−10 m. Using Table \(\PageIndex{1}\) and Table \(\PageIndex{2}\), determine the length of each bond in angstroms.
- a C–C bond
- a C=C bond
- an N≡N bond
- an H–O bond
- Refer to Exercise 3. Why is the nanometer unit useful as a unit for expressing bond lengths?
- Refer to Exercise 4. Why is the angstrom unit useful as a unit for expressing bond lengths?
- Using Figure \(\PageIndex{3}\), determine which atom in each pair has the higher electronegativity.
- H or C
- O or Br
- Na or Rb
- I or Cl
- Using Figure \(\PageIndex{3}\), determine which atom in each pair has the lower electronegativity.
- Mg or O
- S or F
- Al or Ga
- O or I
- Will the electrons be shared equally or unequally across each covalent bond? If unequally, to which atom are the electrons more strongly drawn?
- a C–O bond
- an F–F bond
- an S–N bond
- an I–Cl bond
10. Will the electrons be shared equally or unequally across each covalent bond? If unequally, to which atom are the electrons more strongly drawn?
- a C–C bond
- a S–Cl bond
- an O–H bond
- an H–H bond
11. Arrange the following bonds from least polar to most polar: H-F, H-N, H-O, H-C
12. Arrange the following bonds from least polar to most polar: C-F, C-N, C-O, C-C
Answers
- A C–O bond is longer.
2. An H-N bond is shorter than an H-C bond.
-
- 0.143 nm
- 0.120 nm
- 0.100 nm
- 0.116 nm
4.
- 1.54 Å
- 1.34 Å
- 1.10 Å
- 0.97 Å
- Actual bond lengths are very small, so the nanometer unit makes the expression of length easier to understand.
6. Actual bond lengths are very small, so the angstrom unit makes the expression of length easier to understand.
-
- C
- O
- Na
- Cl
8.
- Mg
- S
- Al
- I
9.
- unequally toward the O
- equally
- unequally toward the N
- unequally toward the Cl
10.
- equally
- unequally toward the Cl
- unequally toward the O
- equally
11. The electronegativity difference increases from 0.4; 0.9; 1.4; 1.9. Hence, the least to most polar: H-C, H-N, H-O, H-F
12. The electronegativity difference increases from 0; 0.5; 1.0; 1.5. Hence, the least to most polar: C-C, C-N, C-O, C-F
4.5: Characteristics of Molecules
Concept Review Exercises
- How do you determine the molecular mass of a covalent compound?
- How do you determine the shape of a molecule?
- How do you determine whether a molecule is polar or nonpolar?
Answers
- The molecular mass is the sum of the masses of the atoms in the formula.
2. The shape of a molecule is determined by the position of the atoms, which in turn is determined by the repulsion of the bonded and lone electron pairs around the central atom.
3. If all the bonds in a molecule are nonpolar, the molecule is nonpolar. If it contains identical polar bonds that are oriented symmetrically opposite each other (linear, trigonal planar or tetrahedral) then the molecule is nonpolar. If it contains polar bonds that don't cancel each other's effects, the molecule is polar.
Exercises
- What is the molecular mass of each compound?
- H2S
- N2O4
- ICl3
- HCl
- What is the molecular mass of each compound?
- O2F2
- CCl4
- C6H6
- SO3
- Aspirin (C9H8O4) is a covalent compound. What is its molecular mass?
- Cholesterol (C27H46O) is a biologically important compound. What is its molecular mass?
- What is the shape of each molecule? State whether it is polar or nonpolar.
- H2S
- COCl2
- SO2
- What is the shape of each molecule? State whether it is polar or nonpolar.
- NBr3
- SF2
- SiH4
- Predict the shape of nitrous oxide (N2O), which is used as an anesthetic. A nitrogen atom is in the center of this three-atom molecule. Is this polar?
- Predict the shape of acetylene (C2H2), which has the two carbon atoms in the middle of the molecule with a triple bond. What generalization can you make about the shapes of molecules that have more than one central atom?
Answers
-
- 34.08 amu
- 92.02 amu
- 233.25 amu
- 36.46 amu
2. What is the molecular mass of each compound?
- 70.00 amu
- 153.81 amu
- 78.12 amu
- 80.06 amu
- 180.17 amu
4. 386.73 amu
-
- bent; polar
- trigonal planar; nonpolar
- bent; polar
6.
- pyramidal; polar
- bent; polar
- tetrahedral; nonpolar
7. linear; polar
8. linear; in a molecule with more than one central atom, the geometry around each central atom needs to be examined.

