5.5: Ionic Nomenclature
- Last updated
- Save as PDF
- Page ID
- 402455

- Anonymous
- LibreTexts
Learning Objectives
- To use the rules for naming ionic compounds
After learning a few more details about the names of individual ions, you will be a step away from knowing how to name ionic compounds. This section begins the formal study of nomenclature, the systematic naming of chemical compounds.
Naming Ions
The name of a monatomic cation is simply the name of the element followed by the word ion. Thus, Na+ is the sodium ion, Al3+ is the aluminum ion, Ca2+ is the calcium ion, and so forth. When creating ionic compounds there are three specific circumstances with individual naming rules: binary ionic compounds, ionic compounds containing a metal with a variable charge (transition metal), and polyatomic ionic compounds. Each will be described below.
Binary Compounds
Binary ionic compounds involve only two separate elements, a metal and a nonmetal, and follow the following general rules.
1. Identify the metal (cation) and the nonmetal (anion).
2. Name the metal by it's elemental name without any changes. Example: Na + = Sodium, Mg 2+ = Magnesium
3. Name the nonmetal by using the first syllable of it's elemental name and adding the ending "ide". Example: Chlorine (Cl-) = Chloride
4. Write the name of the metal first and nonmetal second. Example: Sodium Chloride (NaCl) and Magnesium Chloride (MgCl2)
Metals with Multiple Charges
We have seen that some elements in the transition metals lose different numbers of electrons, producing ions of different charges. Iron, for example, can form two cations, each of which, when combined with the same anion, makes a different compound with unique physical and chemical properties. Thus, we need a different name for each iron ion to distinguish Fe2+ from Fe3+. Therefore, when naming ionic compounds containing a metal that may possess variable charges roman numerals will be placed after the metal name reflecting it's charge. Some common examples can be seen below in Table \(\PageIndex{1}\).
Table \(\PageIndex{1}\): Examples of Ionic Compounds Containing Metals of Variable Charges
| Ionic Formula | Name of Compound |
| FeCl2 | Iron (II) Chloride |
| FeCl3 | Iron (III) Chloride |
| Cu2O | Copper (I) Oxide |
| CuO | Copper (II) Oxide |
Determining the charge, and thus the roman numerals, on the metal ion may be difficult initially. However, keep in mind that you know the nonmetal charge and can determine the value of the metal based on the Ionic Formula. For example, the ionic formula FeCl2 contains 2 chlorine atoms but only 1 iron atom. Since chlorine has a charge of -1 and there are 2 atoms there is an overall -2 charge on the compound. Since there is only 1 iron atom the overall positive charge must be equal to +2 to create a neutral compound and the name is Iron (II) Chloride.
Ionic Compounds Containing Polyatomic Ions
Binary Ionic compounds were compounds containing only two elements that were attracted to each other by electrostatic interactions. However, there is another class of ionic compounds created with multiple elements. Polyatomic ions, also referred to as oxyanions because they often contain oxygen, are created from groups of covalently bound atoms containing an overall charge. Table \(\PageIndex{2}\) lists the names of some common polyatomic ions.
| Nonmetal | Formula of Ion | Name of Ion |
|---|---|---|
| Hydrogen | OH- | Hydroxide Ion |
| Carbon |
CO32- HCO3- CN- CH3COO- |
Carbonate Hydrogen Carbonate (Bicarbonate) Cyanide Acetate |
| Chlorine |
ClO4- ClO3- ClO2- ClO- |
Perchlorate Chlorate Chlorite Hypochlorite |
| Nitrogen |
NH4+ NO3- NO2- |
Ammonium Nitrate Nitrite |
| Sulfur |
SO42- HSO4- SO32- HSO3- |
Sulfate Hydrogen Sulfate (or Bisulfate) Sulfite Hydrogen Sulfite (or Bisulfite) |
| Phosphorous |
PO43- HPO42- H2PO4- PO33- |
Phosphate Hydrogen Phosphate Dihydrogen Phosphate Phosphite |
Note that the most common polyatomic ion form for each element contains the ending "ate". If one oxygen is removed, without changing the charge of the overall compound, the ending is changed to the "ite" ending. Example: Phosphate = PO43- and Phosphite = PO33-. These endings represent the number of oxygen contained in the compound and therefore cannot be removed or dropped from the name when they're incorporated into an ionic compound. Naming ionic compounds will follow these rules.
Naming polyatomic ions:
Identify the cation and the anion of the compound. See rules below if the cation is ammonium.
a. If the cation is a metal without variable charge, name as above with its elemental name.
Examples: NaNO3 = Sodium Nitrate, CaCO3 = Calcium Carbonate
b. If the cation is a metal with variable charge, name as above with the associate roman numeral showing its charge.
Examples: CuCO3 = Copper (II) Carbonate, Fe(OH)3 = Iron (III) Hydroxide
Naming compounds with Ammonium:
Identify the anion associated with the ammonium ion.
a. If the nonmetal is a single element (Ex. N, O, P, or Halogen) then name as before with the first syllable of the element followed by the "ide" ending.
Examples: NH4Cl = Ammonium Chloride, (NH4)2O = Ammonium Oxide.
Note that when writing the formula's of compounds containing polyatomic ions that parenthesis are needed when more than one ion is involved in the compound.
b. If ammonium is present with a second polyatomic ion, name the compound with no changes to either polyatomic ion name.
Examples: NH4NO3 = Ammonium Nitrate, (NH4)2CO3 = Ammonium Carbonate
Example \(\PageIndex{1}\)
Name each ion.
- Ca2+
- S2−
- SO32−
- NH4+
- Cu+
- Answer a
-
the calcium ion
- Answer b
-
the sulfide ion
- Answer c
-
the sulfite ion
- Answer d
-
the ammonium ion
- Answer e
-
the copper(I) ion (copper can form cations with either a 1+ or 2+ charge, so we have to specify which charge this ion has)
Exercise \(\PageIndex{1}\)
Name each ion.
- Fe2+
- Fe3+
- SO42−
- Ba2+
- HCO3−
- Answer a
-
the iron (II)
- Answer b
-
the iron (III)
- Answer c
-
the sulfate ion
- Answer d
-
the barium ion
- Answer e
-
the bicarbonate ion or hydrogen carbonate ion
Example \(\PageIndex{2}\)
Write the formula for each ion.
- the bromide ion
- the phosphate ion
- the copper (II) ion
- the magnesium ion
- Answer a
-
Br−
- Answer b
-
PO43−
- Answer c
-
Cu2+
- Answer d
-
Mg2+
Exercise \(\PageIndex{2}\)
Write the formula for each ion.
- the fluoride ion
- the carbonate ion
- the potassium ion
- Answer a
-
F−
- Answer b
-
CO32-
- Answer d
-
K+
Example \(\PageIndex{3}\)
Name each ionic compound.
- Ca3(PO4)2
- KCl
- CuCl
- SnF2
- Answer a
-
calcium phosphate
- Answer c
-
potassium chloride
- Answer d
-
copper(I) chloride
- Answer e
-
tin(II) fluoride
Exercise \(\PageIndex{3}\)
Name each ionic compound, using both Stock and common systems if necessary.
- ZnBr2
- Fe(NO3)3
- Al2O3
- CuF2
- AgF
- Answer a
-
zinc bromide
- Answer b
-
iron (III) nitrate
- Answer c
-
aluminum oxide
- Answer d
-
copper (II) fluoride
- Answer e
-
silver fluoride
Figure \(\PageIndex{1}\) is a synopsis of how to name simple ionic compounds.
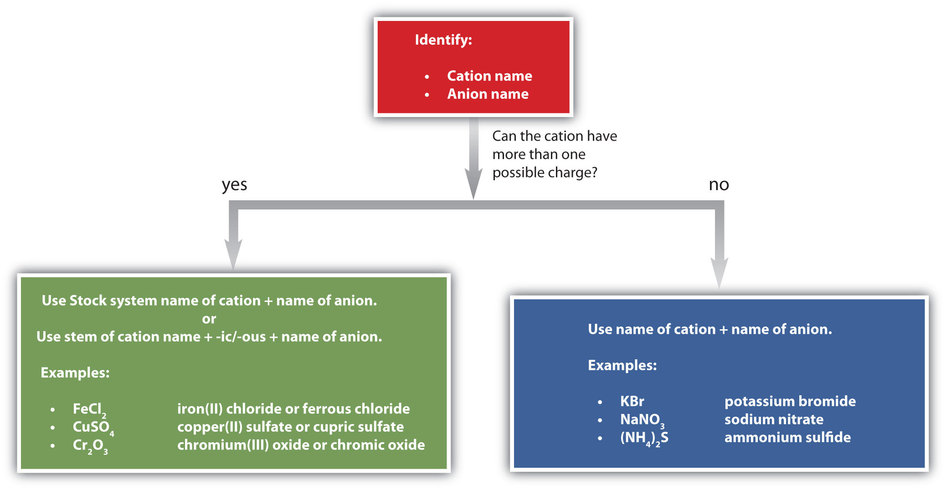
KEY TAKEAWAY
- Each ionic compound has its own unique name that comes from the names of the ions.
EXERCISES
- Briefly describe the process for naming an ionic compound.
- In what order do the names of ions appear in the names of ionic compounds?
3. Which ionic compounds can be named using two different systems? Give an example.
4. Name each ion.
- Ra2+
- P3−
- H2PO4−
- Sn4+
5. Name each ion.
- Cs+
- As3−
- HSO4−
- Sn2+
6. Name the ionic compound formed by each pair of ions.
- Na+ and Br−
- Mg2+ and Br−
- Mg2+ and S2−
7. Name the ionic compound formed by each pair of ions.
- K+ and Cl−
- Mg2+ and Cl−
- Mg2+ and Se2−
8. Name the ionic compound formed by each pair of ions.
- Na+ and N3−
- Mg2+ and N3−
- Al3+ and S2−
9. Name the ionic compound formed by each pair of ions.
- Li+ and N3−
- Mg2+ and P3−
- Li+ and P3−
10. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Fe3+ and Br−
- Fe2+ and Br−
- Au3+ and S2−
- Au+ and S2−
11. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Cr3+ and O2−
- Cr2+ and O2−
- Pb2+ and Cl−
- Pb4+ and Cl−
12. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Cr3+ and NO3−
- Fe2+ and PO43−
- Ca2+ and CrO42−
- Al3+ and OH−
13. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- NH4+ and NO3−
- K+ and Cr2O72−
- Cu+ and CO32−
- Na+ and HCO3−
14. Give two names for each compound.
- Al(HSO4)3
- Mg(HSO4)2
15. Give two names for each compound.
- Co(HCO3)2
- LiHCO3
AnswerS
- Name the cation and then the anion but don’t use numerical prefixes.
- the cation name followed by the anion name
- Ionic compounds in which the cation can have more than one possible charge have two naming systems. FeCl3 is iron(III) chloride.
4.
- the radium ion
- the phosphide ion
- the dihydrogen phosphate ion
- the tin(IV) ion
5.
- the cesium ion
- the arsenide ion
- the hydrogen sulfate ion
- the tin(II) ion
6.
- sodium bromide
- magnesium bromide
- magnesium sulfide
7.
- potassium chloride
- magnesium chloride
- magnesium selenide
8.
- sodium nitride
- magnesium nitride
- aluminum sulfide
9.
- lithium nitride
- magnesium phosphide
- lithium phosphide
10.
- iron(III) bromide
- iron(II) bromide
- gold(III) sulfide
- gold(I) sulfide
11.
- chromium(III) oxide
- chromium(II) oxide
- lead(II) chloride
- lead(IV) chloride
12.
- chromium(III) nitrate
- iron(II) phosphate
- calcium chromate
- aluminum hydroxide
13.
- ammonium nitrate
- potassium dichromate
- copper(I) carbonate
- sodium hydrogen carbonate or sodium bicarbonate
14.
- aluminum hydrogen sulfate or aluminum bisulfate
- magnesium hydrogen sulfate or magnesium bisulfate
15.
- cobalt hydrogen carbonate or cobalt bicarbonate
- lithium hydrogen carbonate or lithium bicarbonate

