5.2.3: Smell and structure of fragrances in soap
- Page ID
- 242464
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Hands-on activity: smell and stereochemistry of organic compounds
Chem0109 Dr. Theis
Before you start, choose a group leader, a record keeper and a technician.
Our noses are powerful analytical tools that can detect the identity and the concentration of substances. In this combined hands-on and research activity, you will explore three organic substances found in plants and often used to add fragrance to soap, lotions, cleaning products and other household items.
Task 1: build a model of a chiral carbon

Take a pea-sized amount of playdough (from your take-home kit) and squeeze it into the shape of a tetrahedron with your two thumbs and index fingers. Take a flat toothpick (from your take-home kit, in one of the tall tubes with blue screw-cap lids), break it into 4 roughly equal pieces, mark the tips of the pieces four different ways, and push one piece each into the four corners of the tetrahedron. Draw the structure of your model, using wedge and dash representation.

would be drawn as:
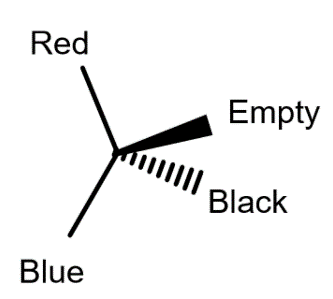
Take your model apart and randomly build it again (e.g. randomly picking the toothpick pieces without looking). Compare your second model with the drawing of the first by rotating it. In the example above, the blue and red group should be in the plane of the paper oriented like in the drawing. Then, determine whether the two are identical or enantiomers by checking which group points at you and which away from you. Draw the second model as well to document your “experiment”.
Task 2: smell samples
In your take-home kit, there are three closed tubes (not filled with liquid: please do not open the tubes with liquids for this activity) with a sample in the lid. Take those three tubes out of the zip lock bag, close the zip lock bag and set it aside. For each closed tube, hold it close to your nose, open the lid and smell (these are household items that are fine to smell like this – in the chemical lab, you would use a technique limiting the amount of substance you inhale). Then, close the lid again. Make a table with your description of your smell perception and with the color of the sample.
Task 3: Identify the functional groups in three flavor compounds
The three samples you just smelled were soap with two different fragrances added, and toothpaste. They contain gamma-nonalactone, limonene and menthol. The skeletal structures of these three substances are shown below.
 |
 |
 |
| γ-nonalactone | limonene | menthol |
Each person in your group will work on one of those (group leader: gamma-nonalactone, record keeper: limonene, technician: menthol). If you are in a group of four: the fourth person will work on 4-hydroxyl nonanoic acid (shown below).

4-hydroxyl nonanoic acid
For each molecule, identify the functional groups present. One of them contains an ester. Esters are condensation products of a hydroxyl (alcohol) and carboxylic acid (fatty acid) group that has the condensed formula RC(=O)OR. Otherwise, you should be familiar with all the functional groups.
Task 4: Find the chiral centers in “your” molecule
All of the four substances are chiral, and some have more than one chiral carbon. Identify all of them.
Task 5: Research health and safety information concerning your molecule
People have a right to know what kind of hazards they are exposed to when handling dangerous substances. The three samples I gave you are not dangerous to touch or smell (but don’t eat soap or toothpaste). However, in their pure form in large quantities, they will be dangerous. Search on the internet for the safety data sheets (SDS or formerly MSDS) for your molecule. The data sheets contain information for health care workers, for the fire department, and for the public. From the information present, find the flammability and the lethal dose (LD50) and jot them down. If no LD50 values are given, try finding them on the internet, writing down the value and the source if you do find information.
Task 6: Share what you found with the record keeper in your group
The record keeper should summarize the findings, and then give them to the group leader, who then writes up a short report.

