5.1.12: Enzyme assay
- Page ID
- 242460
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Lab #12: Enzyme assay (amylase)
Many diagnostic tests are based on enzyme activity. Either the test will determine the concentration of an enzyme in a sample, or it will indirectly determine the concentration of a small molecule in a sample, through a so-called enzyme-linked test (e.g. ELIZA-type test). Today, we will test the activity of the enzyme amylase at different temperatures and pH values.

 Amylase catalyzes the hydrolysis of starch, made up fo glucose building blocks. The product is maltose, a disaccharide. In the activity, we will hydrolize a starch sample with amylase. As the reaction proceeds, we will test for the presence of starch by adding a mixture of potassium iodide and iodine (KI and I2). These form linear I3- and I5- ions embedded in the spiral-staircase structure of starch, with a dark blue color. Once the starch is digested by the enzyme, the color is no longer observed.
Amylase catalyzes the hydrolysis of starch, made up fo glucose building blocks. The product is maltose, a disaccharide. In the activity, we will hydrolize a starch sample with amylase. As the reaction proceeds, we will test for the presence of starch by adding a mixture of potassium iodide and iodine (KI and I2). These form linear I3- and I5- ions embedded in the spiral-staircase structure of starch, with a dark blue color. Once the starch is digested by the enzyme, the color is no longer observed.
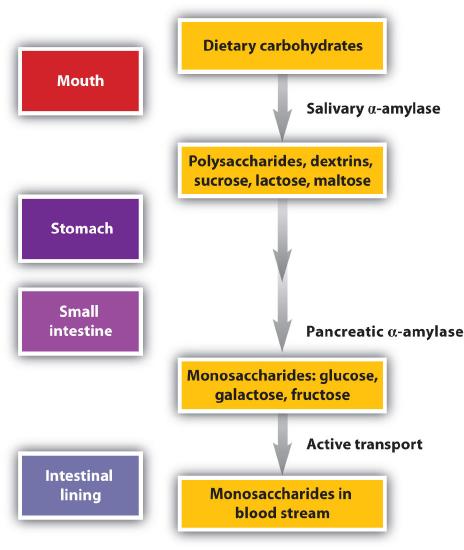 Our amylase sample is from a bacterium. However, you could do a similar experiment at home by using the amylase from human saliva without purification (the easiest source would be your own saliva). Starch starts is broken down into disaccharides in the mouth and in the stomach, and further hydrolized to glucose in the small intestine.
Our amylase sample is from a bacterium. However, you could do a similar experiment at home by using the amylase from human saliva without purification (the easiest source would be your own saliva). Starch starts is broken down into disaccharides in the mouth and in the stomach, and further hydrolized to glucose in the small intestine.
Task 1: Positive and negative control
On two different wells on your spot plate, mix 10 µL iodine indicator solution (tube labelled "I") with 10 µL of 0.5 % starch solution (tube labeled "S") or 1:64 diluted starch solution. Record the colors right after mixing (they will fade over time).
Task 2: Basic enzyme assay
Mix 10 µL of amylase solution (1 mg/mL amylase, tube labelled "E"), 50 µL of buffer solution (ammonium acetate, made in lab last week) in a microcentrifuge tube. Start the timer and add 50 µL of 0.5 % starch solution (tube labeled "S"). Incubate at room temperature, removing 10 µL samples at intervals (e.g. 0, 1, 2, 5, 10 min). The samples are mixed with 10 µL iodine indicator solution (10% povidone diluted 1:100 with 0.2 mol/L HCl) already present on a spot plate. Samples taken at earlier times will be blue, while samples taken at later times will be yellowish if the enzyme has broken down most of the starch.
To analyze your results, compare the colors to the controls of task 1. These are starch solutions mixed with iodine stop solution in the absence of enzyme. The first sample on the left shows undiluted starch solution, while the remainder are 1/4, 1/16, 1/64 dilution, and on the far right, no starch at all. (If you let the samples sit in the light for a while, the brownish color of the iodine stop solution fades while the blackish blue color of the starch : I3- complex is more stable). The undiluted starch solution contains about 0.5 g starch per 100 mL solution, or 0.5%. The standards show that 0.03% starch is still detectable, while 0.008% is below the level of detection in this assay. In other words, if your sample looks like negative control, more than 90% of the starch has been digested. If, on the other hand, it looks as dark as the first (left-most) standard below, no or hardly any starch has been digested.

If you let the samples sit in the light for a while, the brownish color of the iodine stop solution fades while the blackish blue color of the starch : I3- complex is slightly more stable. This is because the free I3- species undergoes photo-degradation (which is why iodine solutions are stored protected from light, e.g. in brown bottles).

Task 3: Change something
Repeat the basic assay, but change one parameter of the assay to answer a question of your choice. One possibility is to run the assay at a different pH, using the buffers we made in lab #11 last week. Other ideas are to do a positive or negative control (especially if there was some kind of unexpected result in task 1), or to change other parameters known to affect the rates of catalyzed reactions.

