2.4 Electron Configurations
- Page ID
- 50021
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element.
Introduction
Before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Every element on the Periodic Table consists of atoms, which are composed of protons, neutrons, and electrons. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within 95% probability. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f orbitals have different sublevels, thus can hold more electrons.
As stated, the electron configuration of each element is unique to its position on the periodic table. The energy level is determined by the period and the number of electrons is given by the atomic number of the element. Orbitals on different energy levels are similar to each other, but they occupy different areas in space. The 1s orbital and 2s orbital both have the characteristics of an s orbital (radial nodes, spherical volume probabilities, can only hold two electrons, etc.) but, as they are found in different energy levels, they occupy different spaces around the nucleus. Each orbital can be represented by specific blocks on the periodic table. The s-block is the region of the alkali metals including helium (Groups 1 & 2), the d-block are the transition metals (Groups 3 to 12), the p-block are the main group elements from Groups 13 to 18, and the f-block are the lanthanides and actinides series.

Using the periodic table to determine the electron configurations of atoms is key, but also keep in mind that there are certain rules to follow when assigning electrons to different orbitals. The periodic table is an incredibly helpful tool in writing electron configurations. For more information on how electron configurations and the periodic table are linked, visit the Connecting Electrons to the Periodic Table module.
Rules for Assigning Electron Orbitals
Occupation of Orbitals
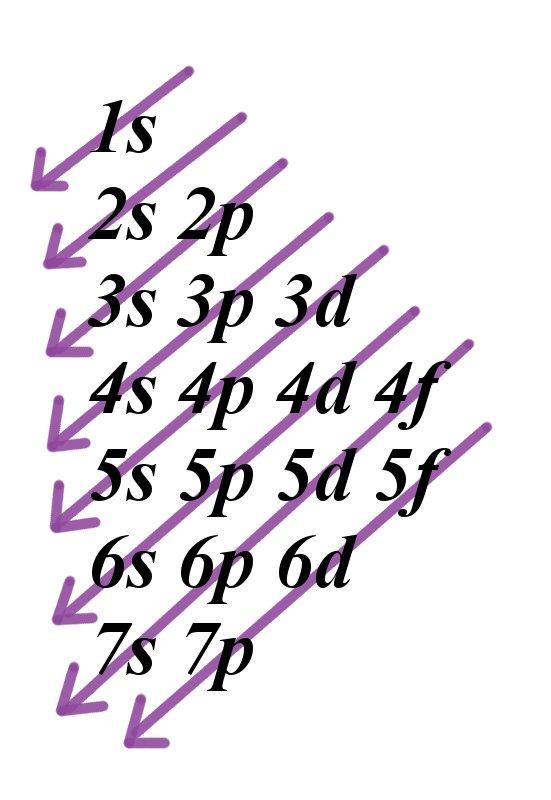
Electrons fill orbitals in a way to minimize the energy of the atom. Therefore, the electrons in an atom fill the principal energy levels in order of increasing energy (the electrons are getting farther from the nucleus). The order of levels filled looks like this:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p
One way to remember this pattern, probably the easiest, is to refer to the periodic table and remember where each orbital block falls to logically deduce this pattern. Another way is to make a table like the one below and use vertical lines to determine which subshells correspond with each other.
Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons can have the same four quantum numbers. The first three (n, l, and ml) may be the same, but the fourth quantum number must be different. A single orbital can hold a maximum of two electrons, which must have opposing spins; otherwise they would have the same four quantum numbers, which is forbidden. One electron is spin up (ms = +1/2) and the other would spin down (ms = -1/2). This tells us that each subshell has double the electrons per orbital. The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f subshell has 7 orbitals with 14 electrons.
Example 1: Hydrogen and Helium
The first three quantum numbers of an electron are n=1, l=0, ml=0. Only two electrons can correspond to these, which would be either ms = -1/2 or ms = +1/2. As we already know from our studies of quantum numbers and electron orbitals, we can conclude that these four quantum numbers refer to the 1s subshell. If only one of the ms values are given then we would have 1s1 (denoting hydrogen) if both are given we would have 1s2 (denoting helium). Visually, this is be represented as:
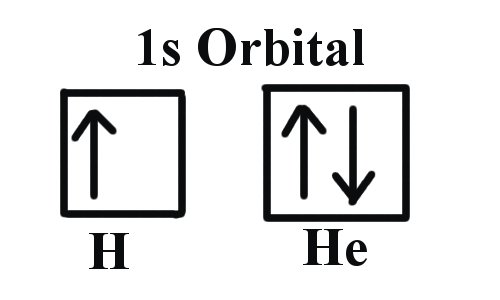
As shown, the 1s subshell can hold only two electrons and, when filled, the electrons have opposite spins.
Hund's Rule
When assigning electrons in orbitals, each electron will first fill all the orbitals with similar energy (also referred to as degenerate) before pairing with another electron in a half-filled orbital. Atoms at ground states tend to have as many unpaired electrons as possible. When visualizing this processes, think about how electrons are exhibiting the same behavior as the same poles on a magnet would if they came into contact; as the negatively charged electrons fill orbitals they first try to get as far as possible from each other before having to pair up.
Example 2: Oxygen and Nitrogen
If we look at the correct electron configuration of the Nitrogen (Z = 7) atom, a very important element in the biology of plants: 1s2 2s2 2p3
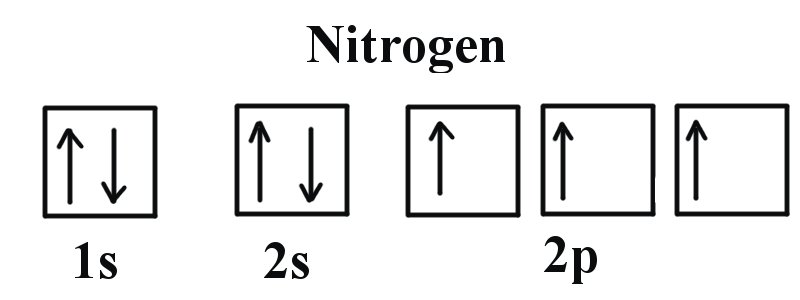
We can clearly see that p orbitals are half-filled as there are three electrons and three p orbitals. This is because Hund's Rule states that the three electrons in the 2p subshell will fill all the empty orbitals first before filling orbitals with electrons in them. If we look at the element after Nitrogen in the same period, Oxygen (Z = 8) its electron configuration is: 1s2 2s2 2p4 (for an atom).
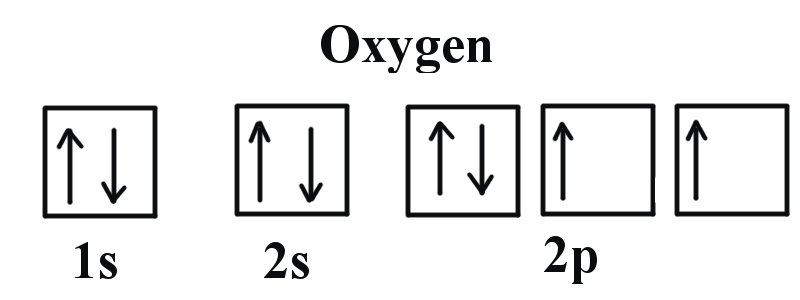
Oxygen has one more electron than Nitrogen and as the orbitals are all half filled the electron must pair up.
The Aufbau Process
Aufbau comes from the German word "aufbauen" meaning "to build." When writing electron configurations, orbitals are built up from atom to atom. When writing the electron configuration for an atom, orbitals are filled in order of increasing atomic number. However, there are some exceptions to this rule.
Example 3: 3rd row elements
Following the pattern across a period from B (Z=5) to Ne (Z=10), the number of electrons increases and the subshells are filled. This example focuses on the p subshell, which fills from boron to neon.
- B (Z=5) configuration: 1s2 2s2 2p1
- C (Z=6) configuration:1s2 2s2 2p2
- N (Z=7) configuration:1s2 2s2 2p3
- O (Z=8) configuration:1s2 2s2 2p4
- F (Z=9) configuration:1s2 2s2 2p5
- Ne (Z=10) configuration:1s2 2s2 2p6
Exceptions
Although the Aufbau rule accurately predicts the electron configuration of most elements, there are notable exceptions among the transition metals and heavier elements. The reason these exceptions occur is that some elements are more stable with fewer electrons in some subshells and more electrons in others (Table 1).
| Period 4: | Period 5: |
| Chromium: Z:24 [Ar] 3d54s1 | Niobium: Z:41 [Kr] 5s1 4d4 |
| Copper: Z:29 [Ar] 3d104s1 | Molybdenum: Z:42 [Kr] 5s1 4d5 |
|
| Ruthenium: Z:44 [Kr] 5s1 4d7 |
|
| Rhodium: Z:45 [Kr] 5s1 4d8 |
|
| Palladium: Z:46 [Kr] 4d10 |
|
| Silver: Z:47 [Kr] 5s1 4d10 |
| Period 6: | Period 7: |
| Lanthanum: Z:57 [Xe] 6s2 5d1 | Actinium: Z:89 [Rn] 7s2 6d1 |
| Cerium: Z:58 [Xe] 6s2 4f1 5d1 | Thorium: Z:90 [Rn] 7s2 6d2 |
| Gadolinium: Z:64 [Xe] 6s2 4f7 5d1 | Protactium: Z:91 [Rn] 7s2 5f2 6d1 |
| Platinum: Z:78 [Xe] 6s1 4f14 5d9 | Uranium: Z:92 [Rn] 7s2 5f3 6d1 |
| Gold: Z:79 [Xe] 6s1 4f14 5d10 | Neptunium: Z:93 [Rn] 7s2 5f4 6d1 |
|
| Curium: Z:96 [Rn] 7s2 5f7 6d1 |
|
| Lawrencium: Z:103 [Rn] 7s2 5f14 7p1 |
Writing Electron Configurations
When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. The total number of electrons is the atomic number, Z. The rules above allow one to write the electron configurations for all the elements in the periodic table. Three methods are used to write electron configurations:
- orbital diagrams
- spdf notation
- noble gas notation
Each method has its own purpose and each has its own drawbacks.
Orbital Diagrams
An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above.
Example 4: Aluminum and Iridium
Write the electron configuration for aluminum and iridium.
SOLUTION
Aluminum is in the 3rd period and it has an atomic number of Z=13. If we look at the periodic table we can see that its in the p-block as it is in group 13. Now we shall look at the orbitals it will fill: 1s, 2s, 2p, 3s, 3p. We know that aluminum completely fills the 1s, 2s, 2p, and 3s orbitals because mathematically this would be 2+2+6+2=12. The last electron is in the 3p orbital. Also another way of thinking about it is that as you move from each orbital block, the subshells become filled as you complete each section of the orbital in the period. The block that the atom is in (in the case for aluminum: 3p) is where we will count to get the number of electrons in the last subshell (for aluminum this would be one electron because its the first element in the period 3 p-block). This gives the following:
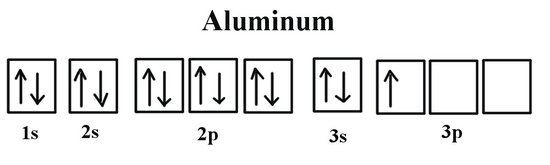
Note that in the orbital diagram, the two opposing spins of the electron can be visualized. This is why it is sometimes useful to think about electron configuration in terms of the diagram. However, because it is the most time consuming method, it is more common to write or see electron configurations in spdf notation and noble gas notation. Another example is the electron configuration of iridium:
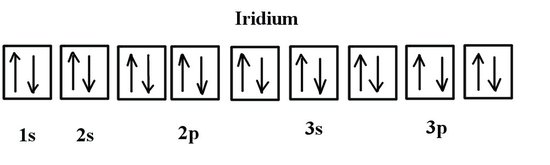
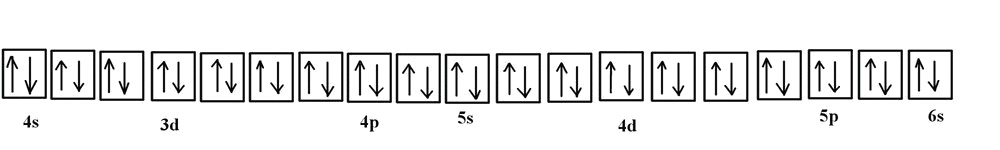
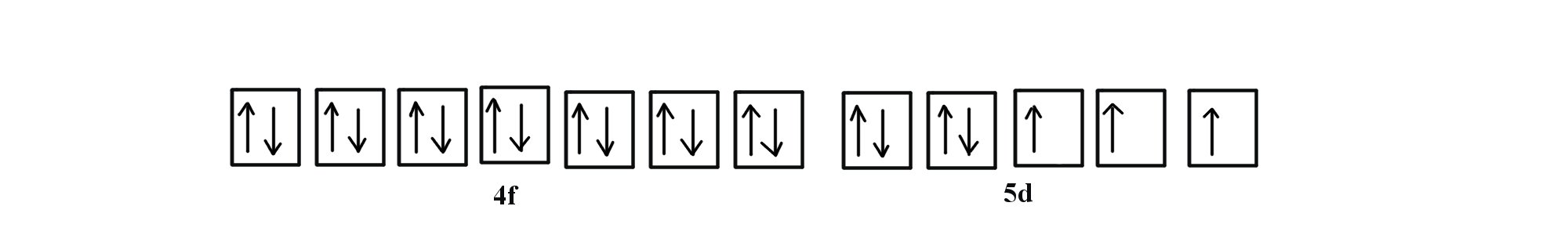
The electron configuration of iridium is much longer than aluminum. Although drawing out each orbital may prove to be helpful in determining unpaired electrons, it is very time consuming and often not as practical as the spdf notation, especially for atoms with much longer configurations. Hund's rule is also followed, as each electron fills up each 5d orbital before being forced to pair with another electron.
spdf Notation
The most common way to describe electron configurations is to write distributions in the spdf notation. Although the distributions of electrons in each orbital are not as apparent as in the diagram, the total number of electrons in each energy level is described by a superscript that follows the relating energy level. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: 1s2. This is the electron configuration of helium; it denotes a full s orbital. The periodic table is used as a reference to accurately write the electron configurations of all atoms.
Example 5: Yttrium
Write the electronic configuration of Yttrium.
SOLUTION
Start with the straightforward problem of finding the electron configuration of the element yttrium. As always, refer to the periodic table. The element yttrium (symbolized Y) is a transition metal, found in the fifth period and in Group 3. In total it has thirty-nine electrons. Its electron configuration is as follows:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d1
This is a much simpler and more efficient way to portray electron configuration of an atom. A logical way of thinking about it is that all that is required is to fill orbitals across a period and through orbital blocks. The number of elements in each block is the same as in the energy level it corresponds. For example, there are 2 elements in the s-block, and 10 elements in the d-block. Moving across, simply count how many elements fall in each block. Yttrium is the first element in the fourth period d-block; thus there is one electron in that energy level. To check the answer, verify that the subscripts add up to the atomic number. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct.
A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3
The reason why this electron configuration seems more complex is that the f-block, the Lanthanide series, is involved. Most students who first learn electron configurations often have trouble with configurations that must pass through the f-block because they often overlook this break in the table and skip that energy level. Its important to remember that when passing the 5d and 6d energy levels that one must pass through the f-block lanthanoid and actinoid series. Keeping this in mind, this "complex" problem is greatly simplified.
Another method (but less commonly used) of writing the spdf notation is the expanded notation format. This is the same concept as before, except that each individual orbital is represented with a subscript. The p, d, and f orbitals have different sublevels. The p orbitals are px, py, and pz, and if represented on the 2p energy with full orbitals would look like: 2px2 2py2 2pz2. The expanded notation for neon (Ne, Z=10) is written as follows:
1s2 2s2 2px2 2py2 2pz2
The individual orbitals are represented, but the spins on the electrons are not; opposite spins are assumed. When representing the configuration of an atom with half filled orbitals, indicate the two half filled orbitals. The expanded notation for carbon is written as follows:
1s2 2s2 2px1 2py1
Because this form of the spdf notation is not typically used, it is not as important to dwell on this detail as it is to understand how to use the general spdf notation.
Noble Gas Notation
This brings up an interesting point about elements and electron configurations. As the p subshell is filled in the above example about the Aufbau principle (the trend from boron to neon), it reaches the group commonly known as the noble gases. The noble gases have the most stable electron configurations, and are known for being relatively inert. All noble gases have their subshells filled and can be used them as a shorthand way of writing electron configurations for subsequent atoms. This method of writing configurations is called the noble gas notation, in which the noble gas in the period above the element that is being analyzed is used to denote the subshells that element has filled and after which the valence electrons (electrons filling orbitals in the outer most shells) are written. This looks slightly different from spdf notation, as the reference noble gas must be indicated.
Example 6: Vanadium
What is the electronic configuration of vanadium (V, Z=23)?
SOLUTION
Vanadium is the transition metal in the fourth period and the fifth group. The noble gas preceding it is argon (Ar, Z=18), and knowing that vanadium has filled those orbitals before it, argon is used as the reference noble gas. The noble gas in the configuration is denoted E, in brackets: [E]. To find the valance electrons that follow, subtract the atomic numbers: 23 - 18 = 5. Instead of 23 electrons to distribute in orbitals, there are 5. Now there is enough information to write the electron configuration:
Vanadium, V: [Ar] 4s2 3d3
This method streamlines the process of distributing electrons by showing the valence electrons, which determine the chemical properties of atoms. In addition, when determining the number of unpaired electrons in an atom, this method allows quick visualization of the configurations of the valance electrons. In the example above, there are a full s orbital and three half filled d orbitals.
References
- Petrucci, Ralph H et al. General Chemistry: Principles & Modern Applications Ninth Edition. , Upper Saddle River, NJ: Pearson Prentice Hall, 2007. Print
- Sherman, Alan, Sharon J. Sherman, and Leonard Russikoff. Basic Concepts of Chemistry Fifth Edition. Boston, MA: Houghton Mifflin Company, 1992. Print.
- IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8.doi:10.1351/goldbook.
- Scerri, Eric R. "The Electron Configuration Model, Quantum Mechanics, and Reduction." The British Journal for the Philosophy of Science 42.3 (1991): 309 -325.
- Ostrovsky, V.N. (2004). On recent discussion concerning quantum justification of the periodic table of the elements. Foundations of Chemistry, 75, 93-116.
- Meek, T.L., & Allen, L.C. (2002). Configuration irregularities: deviations from the madelung rule and inversion of orbital energy levels. ScienceDirect, 362(5), 362-364.
Problems
Unless specified, use any method to solve the following problems. Answers are given in noble gas notation.
1. Find the electron configurations of the following:
- silicon
- tin
- lead
2. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work.
- Find the electron configuration of iodine
- How many unpaired electrons does iodine have?
3. Thought Questions:
- In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry.
- Describe the major concepts (Hunds, Pauli...etc.) and explain why each is a key part of the "tool kit" when describing electron configurations
- Why is it possible to abbreviate electron configurations with a noble gas in the noble gas notation?
4. Identify the following elements:
- 1s2 2s2 2p6 3s2 3p6 4s2 3d6
- 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7
- 1s2 2s2 2p6 3s2 3p4
- 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p4
5. Without using a periodic table or any other references, fill in the correct box in the periodic table with the letter of each question. (a)The element with electron configuration: 1s2 2s2 2p6 3s2 3p5; (b)A noble gases with f electrons; (c) a fifth-period element whose atoms have three unpaired p electrons; (d) First rowtransition metals having one 4s electron.
Answers
1. Find the electron configuration of the following:
a) silicon: [Ne] 3s2 3p2
b) tin: [Kr] 5s2 4d10 5p2
c) lead: [Xe] 6s2 4f14 5d10 6p2
2. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work.
a) Find the electron configuration of iodine
[Kr] 5s2 4d10 5p5
b) How many unpaired electrons does iodine have?
To find the answer we refer to part a) and look at the valence electrons. We see that iodine has 5 electrons in the p orbitals. We know that the full p orbitals will add up to 6. Using the Hund's rule and Pauli exclusion principals we can make a diagram like the following:
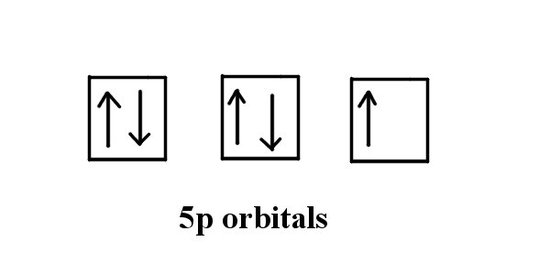
The answer is one.
3. Thought Questions:
a) In your own words describe how to write an electron configuration and why it is an important skill in the study of chemistry.
The first part of this question is straightforward. The second part is slightly more complicated. Because each individual's knowledge of chemistry differs, there are many answers to this question. The important aspect is that we realize that knowing electron configurations helps us determine the valence electrons on an atom. This is important because valence electrons contribute to the unique chemistry of each atom.
b) Describe the major concepts (Hunds, Pauli...etc.) and explain why each is a key part of the "tool kit" when describing electron configurations
This should also be a straightforward question, and if it seems a little difficult refer to the body of this text about these rules and how they relate to creating an electron configuration. Remember to make logical connections! We know that the main "tools" we have in writing electron configurations are orbital occupation, the Pauli exclusion principle, Hund's rule, and the Aufbau process. Orbitals are occupied in a specific order, thus we have to follow this order when assigning electrons. The Pauli exclusion principle states that no two electrons can have the same four quantum numbers . The fourth quantum number, which refers to spin, denotes one of two spin directions. This means that in one orbital there can only be two electrons and they mus have opposite spins. This is important when describing an electron configuration in terms of the orbital diagrams. Hund's rule states that electrons first occupy the similar energy orbitals that are empty before occupying those that are half full. This is especially helpful when determining unpaired electrons. The Aufbau process denotes the method of "building up" each subshell before moving on to the next; we first fill the 2s orbitals before moving to the 2p orbitals.
c) Why is it possible to abbreviate electron configurations with a noble gas in the noble gas notation?
We know that the noble gas has all of its orbitals filled; thus it can be used as a "shorthand" or abbreviated method for writing all of the electron configurations after 1s.
4. Identify the following elements:
a) 1s2 2s2 2p6 3s2 3p6 4s2 3d6
The element is iron, Fe
b) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7
The element is Rhodium, Rh
c) 1s2 2s2 2p6 3s2 3p4
The element is Sulfur, S
d) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p4
The element is Polonium, Po
5. Without using a periodic table or any other references, fill in the correct box in the periodic table with the letter of each question. (a) The element with electron configuration: 1s2 2s2 2p6 3s2 3p5; (b)A noble gases with f electrons; (c) a fifth-period element whose atoms have three unpaired p electrons; (d) First row transition metals having one 4s electron.
Contributors
- Sarah Faizi (University of California Davis)

