11.E: Organic Chemistry (Exercises)
- Page ID
- 83139
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)12: Organic Chemistry
Exercises
Green text involves IUPAC naming.
-
Classify each compound as organic or inorganic. (A) C3H8O; (B) CaCl2; (C) Cr(NH3)3Cl3; (D) C30H48O3N
-
Which compound is likely organic and which is likely inorganic? (A) a flammable compound that boils at 80°C and is insoluble in water; (B) a compound that does not burn, melts at 630°C, and is soluble in water
-
Which member of each pair has a higher melting point? (A) CH3OH and NaOH; (B) CH3Cl and CH3OH; (C) CH3Cl and CH3CH2CH2CH2CH2Cl
-
Use the general formulas for alkanes to write the molecular formula of the alkane with 12 carbon atoms. What would the formula for an alkene with 12 carbons (just one double bond).
-
In alkanes, can there be a two-carbon branch off the second carbon atom of a four-carbon chain? Explain.
-
Indicate whether the structures in each set represent the same compound, isomers, or different. (Naming them will help.)
A) and
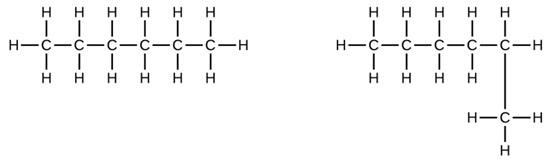
B) CH3CH2CH2CH2CH3 and
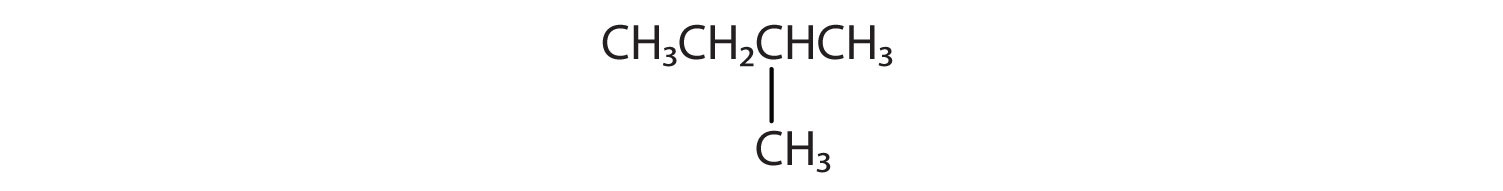
-
Name and write the condensed structural formula for the structural formula to the right.
-
Draw a line-angle formula for the compound CH3CH2CH(CH3)CH2CH2CH3. (Also give the IUPAC name.)
-
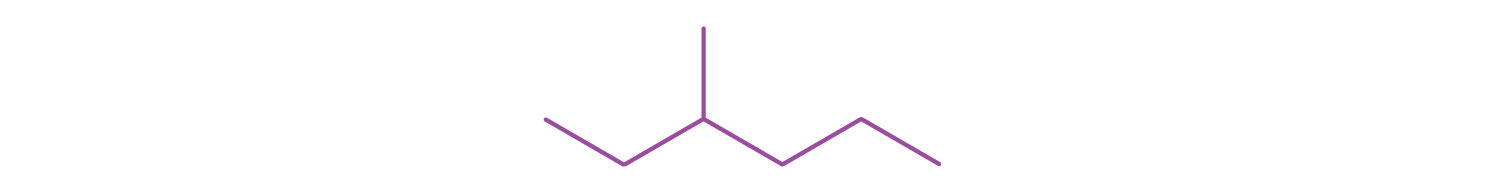
Write the structural formula for the compound represented by this line-angle formula. (Also give the IUPAC name.)

-
Draw the structure for each name. A) 4-ethyl-2-methylhexane; B) 2,2,3,3-tetramethylbutane
-
What is wrong with each name? (Hint: first write the structure as if it were correct.) Give the correct name for each compound. A) 2-dimethylpropane; B) 2,3,3-trimethylbutane; C) 2,4-diethylpentane; D) 3,4-dimethyl-5-propylhexane
-
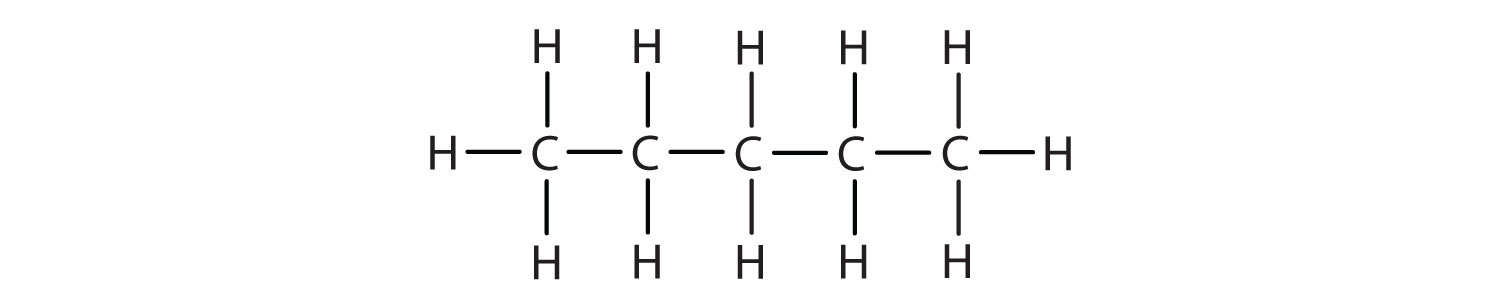
Draw the structures for the five isomeric hexanes (C6H14). Name each by the IUPAC system.
-
Which functional group is present in each of the following? Which are also acidic or basic? A) CH3NHCH2CH3; B) CH3CO2CH3; C) CH3CO2H; D) (CH3)2CHOH
-
Is each molecule cis, trans, or neither? Also give the IUPAC name (cis- or trans- goes in front of name, when appropriate).

Answers
-
A) organic; B) inorganic; C) inorganic; D) organic
-
A) organic; B) inorganic
-
Which member of each pair has a higher melting point? (A) NaOH; (B) CH3OH; (C) CH3CH2CH2CH2CH2Cl
-
alkane C12H26 ; alkene C12H24
-
No; both are five-carbon continuous chains.
-
A) same - both pentane; B) isomers - pentane and 2-methylbutane
-
CH3CH2CH2CH2CH3
-
3-methylhexane
-
2,4-dimethylhexane, (CH3)2CHCH2CH(CH3)CH2CH3
-
A)
 B)
B) 
-
A) Two numbers are needed to indicate two substituents; 2,2-dimethylpropane. B) The lowest possible numbers were not used; 2,2,3-trimethylbutane. C) An ethyl substituent is not possible on the second carbon atom; 3,5-dimethylheptane. D) A propyl substituent is not possible on the fifth carbon atom; 3,4,5-trimethyloctane.
-
CH3CH2CH2CH2CH2CH3; hexane

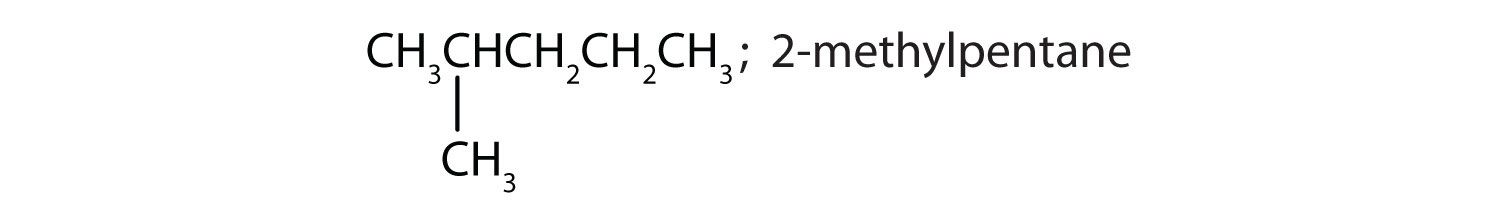


13. A) amine & base; B) ester; C) carboxylic acid & acid; D) alcohol
14. A) cis-8-chloro-2-nonene; B) 3-ethyl-2-pentene is not cis or trans because both groups single bonded to carbon #3 are the same; C) 1,1,-dibromocylcopropane is not cis or trans because both bromine substituents are bonded to the same carbon; D) cis-1,2-dimethylcylcopentane

