14.E: Exercises
- Page ID
- 83212
Additional Exercises
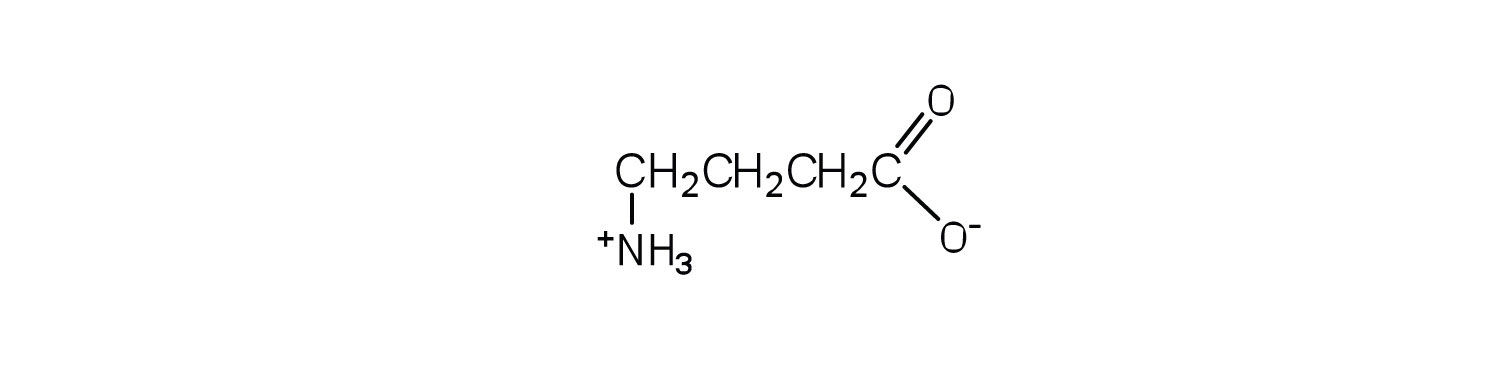
- Below is the structure of the amino acid γ-aminobutyric acid (GABA). Would you expect to find GABA in the amino acid sequence of a protein? Explain.
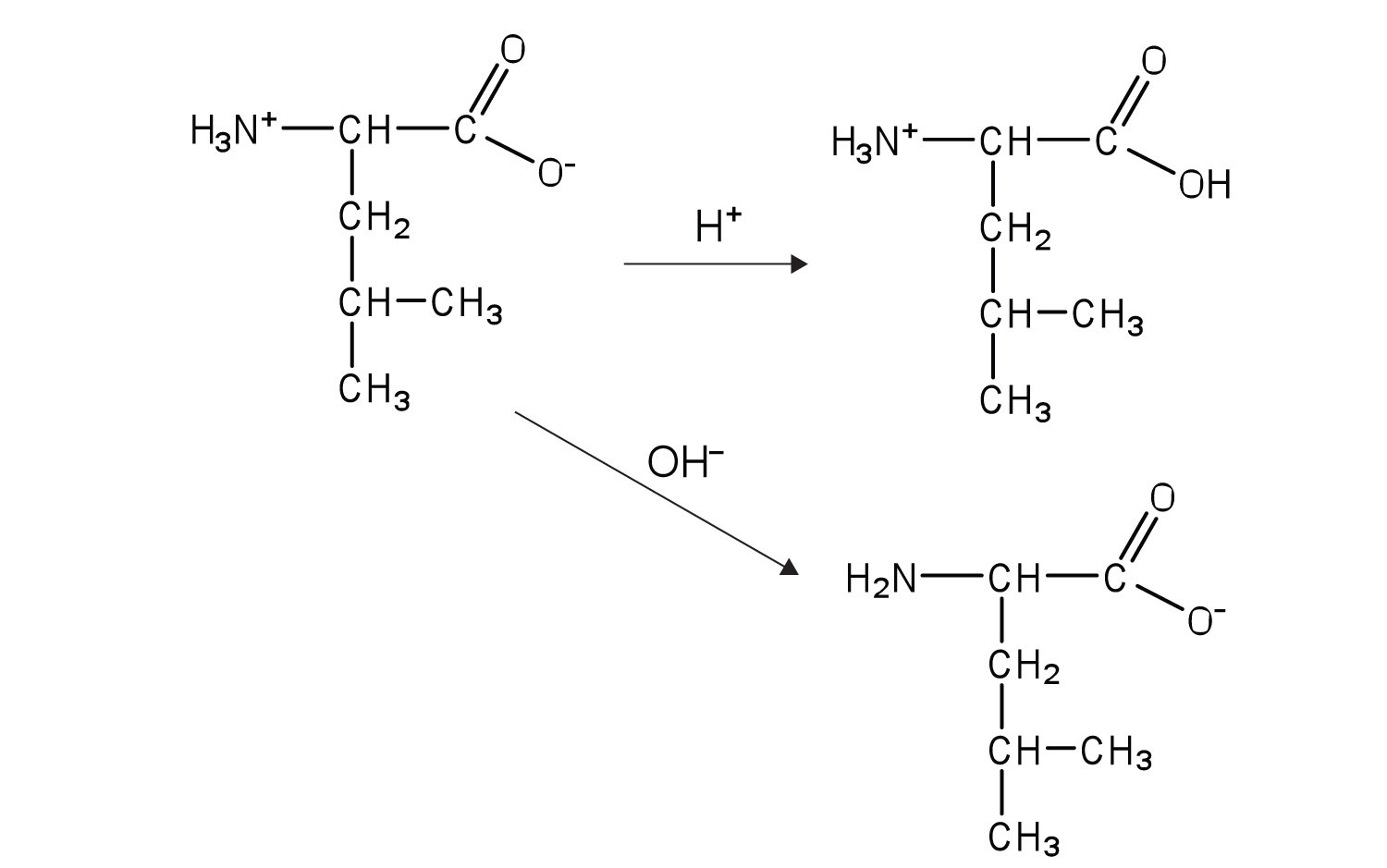
- Write equations to show how leucine can act as a buffer (that is, how it can neutralize added acid or base).
- Bradykinin is a peptide hormone composed of nine amino acids that lowers blood pressure. Its primary structure is arg-pro-pro-gly-phe-ser-pro-phe-arg. Would you expect bradykinin to be positively charged, negatively charged, or neutral at a pH of 6.0? Justify your answer.
- The enzyme lysozyme has an aspartic acid residue in the active site. In acidic solution, the enzyme is inactive, but activity increases as the pH rises to around 6. Explain why.
- A patient has a fever of 39°C. Would you expect the activity of enzymes in the body to increase or decrease relative to their activity at normal body temperature (37°C)?
- Which mutations would have more impact on the function of a protein, the substitution of lysine for leucine or the substitution of serine for threonine? Explain your reasoning.
Answers
-
This amino acid would not be found in proteins because it is not an α-amino acid (which all have general formula +H3NCH(R)CO2-, where the amine and carboxyl group are both directly attached to the same alpha carbon).
-
If acid is added, the extra protons react with the negative carboxyl groups of the amino acid. If base is added, it will react with an H+ from the protonated amine group.
-
Bradykinin would be positively charged; all of the amino acids in that chain except for arginine have R groups that do not become either positively or negatively charged. The two arginines have positively charged at neutral pH, so the peptide would have an overall positive two charge.
-
The enzyme is active when the carboxyl group in the R group (CH2COOH) of aspartic acid does not have the H+ attached (forming CH2COO–); the proton is removed when the solution becomes less acidic, around pH 6 or higher.
-
Normally, reaction rates increase at higher temperatures because the reactants have more energy to overcome the activation energy barrier. With enzymes, however, the activity of enzymes in the body would decrease at higher temperature because the enzymes begin to denature.
-
Substitution of lysine for leucine would have more impact because the change from an amino acid with a nonpolar side chain to one that has a positively charged side chain is more drastic. Both serine and threonine, on the other hand, have polar side chains containing the OH group, so they would behave similarly.

