10.2 Brønsted-Lowry Definition of Acids and Bases
- Page ID
- 83115
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Recognize a compound as a Brønsted-Lowry acid or a Brønsted-Lowry base.
- Illustrate the proton transfer process that defines a Brønsted-Lowry acid-base reaction.
Ammonia (NH3) increases the hydroxide ion concentration in aqueous solution by reacting with water rather than releasing hydroxide ions directly. In fact, the Arrhenius definitions of an acid and a base focus on hydrogen ions and hydroxide ions. Are there more fundamental definitions for acids and bases?
In 1923, the Danish scientist Johannes Brønsted and the English scientist Thomas Lowry independently proposed new definitions for acids and bases. Rather than considering both hydrogen and hydroxide ions, they focused on the hydrogen ion only. A Brønsted-Lowry acid is a compound that supplies a hydrogen ion in a reaction. A Brønsted-Lowry base, conversely, is a compound that accepts a hydrogen ion in a reaction. Thus, the Brønsted-Lowry definitions of an acid and a base focus on the movement of hydrogen ions in a reaction, rather than on the production of hydrogen ions and hydroxide ions in an aqueous solution.
Let us use the reaction of ammonia in water to demonstrate the Brønsted-Lowry definitions of an acid and a base. Ammonia and water molecules are reactants, while the ammonium ion and the hydroxide ion are products:
\[NH_{3(aq)} + H_2O_{(ℓ)} \rightarrow NH^+_{4(aq)} + OH^−_{(aq)} \label{Eq1}\]
What has happened in this reaction is that the original water molecule has donated a hydrogen ion to the original ammonia molecule, which in turn has accepted the hydrogen ion. We can illustrate this as follows:
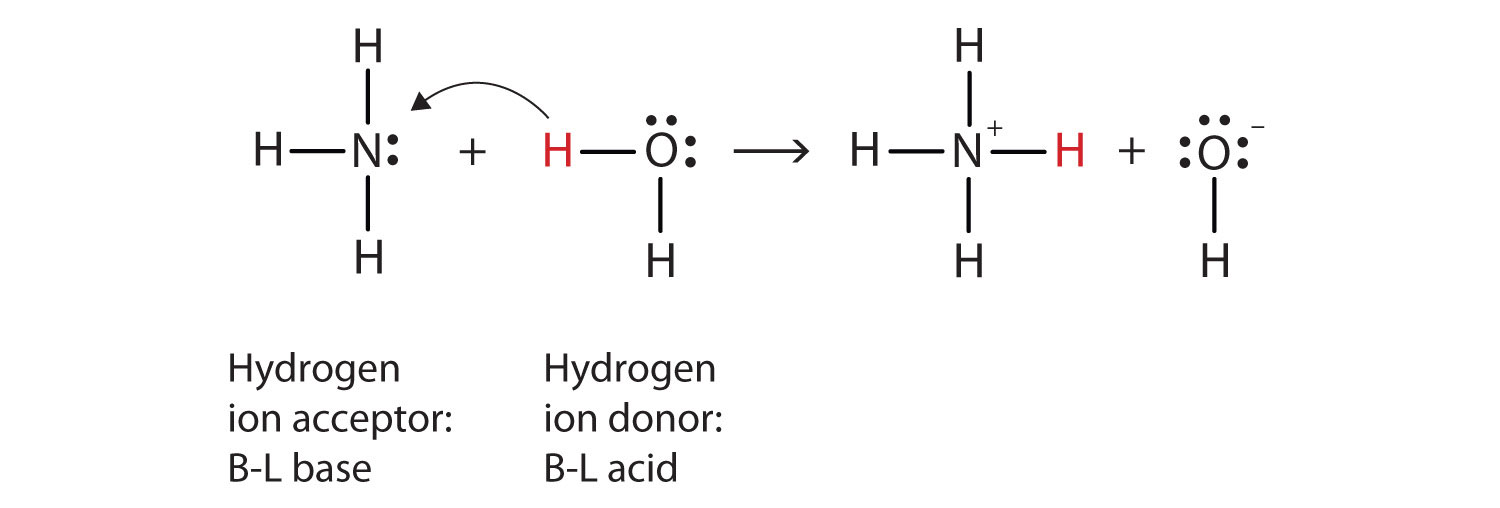
Because the water molecule donates a hydrogen ion to the ammonia, it is the Brønsted-Lowry acid, while the ammonia molecule—which accepts the hydrogen ion—is the Brønsted-Lowry base. Thus, ammonia acts as a base in both the Arrhenius sense and the Brønsted-Lowry sense.
Is an Arrhenius acid like hydrochloric acid still an acid in the Brønsted-Lowry sense? Yes, but it requires us to understand what really happens when HCl is dissolved in water. Recall that the hydrogen atom is a single proton surrounded by a single electron. To make the hydrogen ion, we remove the electron, leaving a bare proton. Do we really have bare protons floating around in aqueous solution? No, we do not. What really happens is that the H+ ion attaches itself to H2O to make H3O+, which is called the hydronium ion. For most purposes, H+ and H3O+ represent the same species, but writing H3O+ instead of H+ shows that we understand that there are no bare protons floating around in solution. Rather, these protons are actually attached to solvent molecules.
A proton in aqueous solution may be surrounded by more than one water molecule, leading to formulas like H5O2+ or H9O4+ rather than H3O+. It is simpler, however, to use H3O+.
With this in mind, how do we define HCl as an acid in the Brønsted-Lowry sense? Consider what happens when HCl is dissolved in H2O:
\[HCl_{(g)} + H_2O_{(ℓ)} \rightarrow H_3O^+_{(aq)} + Cl^−_{(aq)} \label{Eq2}\]
We can depict this process using Lewis electron dot diagrams:
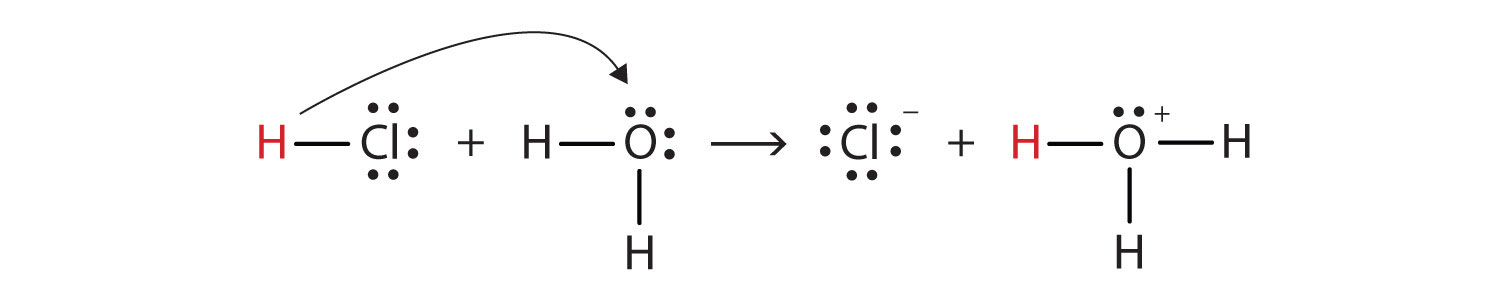
Now we see that a hydrogen ion is transferred from the HCl molecule to the H2O molecule to make chloride ions and hydronium ions. As the hydrogen ion donor, HCl acts as a Brønsted-Lowry acid; as a hydrogen ion acceptor, H2O is a Brønsted-Lowry base. So HCl is an acid not just in the Arrhenius sense but also in the Brønsted-Lowry sense. Moreover, by the Brønsted-Lowry definitions, H2O is a base in the formation of aqueous HCl. So the Brønsted-Lowry definitions of an acid and a base classify the dissolving of HCl in water as a reaction between an acid and a base—although the Arrhenius definition would not have labeled H2O a base in this circumstance.
All Arrhenius acids and bases are Brønsted-Lowry acids and bases as well. However, not all Brønsted-Lowry acids and bases are Arrhenius acids and bases.
CONJUGATE BASE AND ACID
After the acid loses a proton, it is often referred to as the conjugate base. For example, if the monoprotic acid HCl loses a proton, it becomes Cl-. The Cl- no longer has a proton so is no longer an acid, so we call it the conjugate base. An acid like H2SO4, is called diprotic because it has two protons that can be donated. After losing one proton, H2SO4 becomes HSO4-. The HSO4- is still called a conjugate base because it was formed from an acid and will be less acidic than H2SO4.
After the base loses a proton, it is often referred to as the conjugate base. For example, if NH3 base gains a proton, it becomes NH4+. The NH4+ now has extra protons and is more acidic, so we call it the conjugate acid.
Below are two examples that label the acid, base, conjugate acid and conjugate base for each.
NH3 + H2O → NH4+ + OH-
base acid conjugate acid conjugate base
HCl + H2O → Cl- + H3O+
acid base conjugate base conjugate acid
Example \(\PageIndex{1}\)
Aniline (C6H5NH2) is slightly soluble in water. It has a nitrogen atom that can accept a hydrogen ion from a water molecule just like the nitrogen atom in ammonia does. Write the chemical equation for this reaction and identify the Brønsted-Lowry acid and base, plus conjugates.
SOLUTION
C6H5NH2 and H2O are the reactants. When C6H5NH2 accepts a proton from H2O, it gains an extra H and a positive charge and leaves an OH− ion behind. The reaction is as follows:
C6H5NH2(aq) + H2O(ℓ) → C6H5NH3+(aq) + OH−(aq)
Because C6H5NH2 accepts a proton, it is the Brønsted-Lowry base. The C6H5NH3+ product is therefore the conjugate acid.
The H2O molecule, because it donates a proton, is the Brønsted-Lowry acid. The OH− product is therefore the conjugate base.
The Brønsted-Lowry definitions of an acid and a base can be applied to chemical reactions that occur in solvents other than water. The following example illustrates.
Example \(\PageIndex{2}\)
Sodium amide (NaNH2) dissolves in methanol (CH3OH) and separates into sodium ions and amide ions (NH2−). Normally alcohols, like methanol, are not significantly acidic. However in the presence of an extremely strong base, like the amide anion, alcohols will behave as acids. Write a balanced chemical equation for the reaction NH2− with CH3OH. Identify the Brønsted-Lowry acid and base, plus conjugates. Hint: Pay attention to changes in charges as the proton (H+) is exchanged.
SOLUTION
The equation for the reaction is between NH2− and CH3OH to make NH3 and CH3O− is as follows:
NH2−(solv) + CH3OH(ℓ) → NH3(solv) + CH3O−(solv)
The label (solv) indicates that the species are dissolved in some solvent, in contrast to (aq), which specifies an aqueous (H2O) solution.
In this reaction, NH2− gains H+ to make NH3. As the proton acceptor, NH2− is the Brønsted-Lowry base. The NH3 product is therefore the conjugate acid.
CH3OH loses H+ to make CH3O−. As the proton donor, CH3OH is the Brønsted-Lowry acid. The CH3OH− product is therefore the conjugate base.
To Your Health: Brønsted-Lowry Acid-Base Reactions in Pharmaceuticals
There are many interesting applications of Brønsted-Lowry acid-base reactions in the pharmaceutical industry. For example, drugs often need to be water soluble for maximum effectiveness. However, many complex organic compounds are not soluble or are only slightly soluble in water. Fortunately, those drugs that contain proton-accepting nitrogen atoms (and there are a lot of them) can be reacted with dilute hydrochloric acid [HCl(aq)]. The nitrogen atoms—acting as Brønsted-Lowry bases—accept the hydrogen ions from the acid to make an ion, which is usually much more soluble in water. The modified drug molecules can then be isolated as chloride salts:
\[\mathrm{RN(sl\: aq) + H^+(aq) \rightarrow RNH^+(aq) \xrightarrow{Cl^-(aq)} RNHCl(s)} \label{Eq3}\]
where RN represents some organic compound containing nitrogen. The label (sl aq) means “slightly aqueous,” indicating that the compound RN is only slightly soluble. Drugs that are modified in this way are called hydrochloride salts. Examples include the powerful painkiller codeine, which is commonly administered as codeine hydrochloride. Acids other than hydrochloric acid are also used. Hydrobromic acid, for example, gives hydrobromide salts. Dextromethorphan, an ingredient in many cough medicines, is dispensed as dextromethorphan hydrobromide. The accompanying figure shows another medication as a hydrochloride salt.
The name of this medicine makes it clear that it exists as the hydrochloride salt. Figure used with permission from Wikipedia.
Concept Review Exercise
-
Give the definitions of a Brønsted-Lowry acid and a Brønsted-Lowry base.
Answer
-
A Brønsted-Lowry acid is a proton donor, while a Brønsted-Lowry base is a proton acceptor.
Key Takeaways
- A Brønsted-Lowry acid is a proton donor, and a Brønsted-Lowry base is a proton acceptor.
- Brønsted-Lowry acid-base reactions are essentially proton transfer reactions.

