21.S: Nuclear Chemistry (Summary)
- Page ID
- 91342
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)21.1: Radioactivity
-
- nucleons – neutron and proton
- all atoms of a given element have the same number of protons, atomic number
- isotopes – atoms with the same atomic number but different mass numbers
- three isotopes of uranium: uranium-233, uranium-235, uranium-238
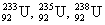 (superscript is mass number, subscript atomic number)
(superscript is mass number, subscript atomic number)- radionuclides – nuclei that are radioactive
- radioisotopes – atoms containing radionuclides
21.1.1 Nuclear Equations
-
- alpha particles – helium-4 particles
- alpha radiation – stream of alpha particles
- emission of radiation is one way that an unstable nucleus is transformed into a more stable one

- superscript = mass number
- subscript = atomic number
- radioactive decay – when a nucleus spontaneously decomposes
- sum of the mass numbers is the same on both sides of the equation
- sum of the atomic numbers same on both sides of the equation
- radioactive properties of the nucleus are independent of the state of chemical combination of the atom
- chemical form does not matter when writing nuclear equations
21.1.2 Types of Radioactive Decay
- three most common type of radioactive decay: alpha(α), beta(β), and gamma(γ) radiation
| Property | α | β | γ |
|---|---|---|---|
| Charge | 2+ | 1- | 0 |
| Mass | 6.64x10-24 g | 9.11x10-28 g | 0 |
| Relative penetrating power | 1 | 100 | 10,000 |
| Nature of radiation | electrons | High-energy photons |
-
- beta particles – high speed electrons emitted by an unstable nucleus
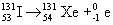
- beta decay results in increasing the atomic number

- gamma radiation – high-energy protons
- gamma radiation does not change atomic number or mass number or a nucleus
- almost always accompanies other radioactive emission
- represents the energy lost when the remaining nucleons reorganize into more stable arrangements
- positron – particle that has same mass as an electron but opposite charge
- represented by
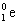
- emission of a positron has effect of converting a proton to a neutron decreasing atomic number of nucleus by 1
- electron capture – the capture by the nucleus of an inner-shell electron from the electron cloud surrounding the nucleus
- has effect of converting a proton to neutron
| Particle | Symbol |
|---|---|
| Neutron | |
| Proton | |
| Electron | |
| Alpha Particle | |
| Beta Particle | |
| Positron |
21.2: Patterns of Nuclear Stability
21.2.1 Neutron-to-Proton Ratio
-
- strong nuclear force – a strong force of attraction between a large number of protons in the small volume of the nucleus
- stable nuclei with low atomic numbers up to 20 have nearly equal number of neutrons and protons
- for higher atomic numbers, the number of neutrons are greater than the number of protons
- the neutron-to-proton ratio of stable nuclei increase with increasing atomic number
- belt of stability – area where all stable nuclei are found
- ends at bismuth
- all nuclei with 84 or more protons are radioactive
- an even number of protons and neutrons is more stable than an odd number
- determining type of radioactive decay
- 1) nuclei above the belt of stability
- high neutron-to-proton ratios
- move toward belt of stability by emitting a beta particle
- decreases the number of neutrons and increases the number of protons in a nucleus
- 2) nuclei below the belt of stability
- low neutron-to-proton ratios
- move toward belt of stability by positron emission or electron capture
- increase number of neutrons and decrease the number of protons
- positron emission more common with lower nuclear charges
- electron capture becomes more common with increasing nuclear charge
- 3) nuclei with atomic numbers
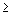 84
84 - alpha emission
- decreases both number of neutrons and protons by 2
21.2.2 Radioactive Series
-
- some nuclei cannot game stability by a single emission
- radioactive series or nuclear disintegration series – series of nuclear reactions that begin with an unstable nucleus to a stable one
- three types of radioactive series found in nature
- uranium-238 to lead-206, uranium-235 to leat-207, and thorium-232 to lead-208
21.2.3 Further Observations
-
- nuclei with 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82, or 126 neutrons are more stable than with nuclei without these numbers
- numbers called magic numbers
- nuclei with even number of protons and neutrons more stable than with odd number of protons and neutrons
- observations made in terms of the shell model of the nucleus
- nucleons reside in shells
- magic numbers represent closed shells in nuclei
21.3: Nuclear Transmutations
-
- nuclear transmutations – nuclear reactions caused by the collision of one nucleus with a neutron or by another nucleus
- first conversion of one nucleus into another performed by Ernest Rutherford in 1919
- converted nitrogen-14 to oxygen-17


21.3.1 Using Charged Particles
-
- particle accelerators – used to accelerate particles at very high speeds
- cyclotron, and synchrotron
21.3.2 Using Neutrons
- neutrons do not need to be accelerated
21.3.4 Transuranium Elements
- transuranium elements – elements with atomic numbers above 92 that are produced by artificial transmutations
21.4: Rates of Radioactive Decay
-
- radioactive decay is a first-order process
- has characteristic of half life, which is the time required for half of any given quantity of a substance to react
- half-life unaffected by external conditions
21.4.1 Dating
-
- radiocarbon dating assumes that the ratio of carbon-14 to carbon-12 in the atmosphere has been constant for at least 50,000 years
- age of rocks can be determined by ratio of uranium-238 to lead-206
21.4.2 Calculations Based on Half-life
-
- rate = kN
- k = decay constant, N = nuclei
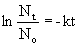
- t = time interval of decay, k = decay constant, N0 = initial number of nuclei at time zero, Nt = number remaining after time interval

21.5 Detection of Radioactivity
-
- Geiger counter – device used to measure and detect radioactivity
- Based on ionization of matter caused by radiation
- Phosphors – substances that give off light when exposed to radiation
- Scintillation counter – used to detect and measure radiation based on tiny flashes of light produced when radiation strikes a suitable phosphor
21.5.1 Radiotracers
-
- radioisotopes can be used to follow an element through its chemical reactions
- isotopes of same element have same properties
- radiotracer – radioisotopes used to trace an element
21.6: Energy Changes in Nuclear Reactions
-

- E = energy, m = mass, c = speed of light
- If system loses mass, it loses energy (exothermic)
- If system gains mass, it gains energy (endothermic)
21.6.1 Nuclear Binding Energies
-
- masses of nuclei always less than masses of individual nucleons
- mass defect – mass difference between a nucleus and its constituent nucleons
- energy is needed to break nucleus into separated protons and neutrons, addition of energy must also have an increase in mass
- nuclear binding energy – energy required to separate a nucleus into its individual nucleons
- the larger to nuclear binding energy the more stable the nucleus toward decomposition
- fission – energy produced when heavy nuclei split
- fusion – energy produced when light nuclei fuse
21.7: Nuclear Fission
- fission and fusion both exothermic
- chain reaction – reaction in which the neutrons produced in one fission cause further fission reactions
- in order for a fission chain reaction to occur, the sample of fissionable material must have a certain minimum mass
- critical mass – amount of fissionable material large enough to maintain the chain reaction with a constant rate of fission
- supercritical mass – mass in excess of a critical mass
21.7.1 Nuclear Reactors
- nuclear reactors the fission is controlled to generate a constant power
- reactor core consists of fissionable fuel, control rods, a moderator, and cooling fluid
- fission products are extremely radioactive and are thus hard to store
- about 20 half-lives needed for products to react acceptable levels for biological exposure
21.8: Nuclear Fusion
- fusion is appealing because of availability of light isotopes and fusion products are not radioactive
- high energies needed to overcome attraction of nuclei
- thermonuclear reactions – fusion reactions
- lowest temperature required is about 40,000,000 K
21.9: Biological Effects of Radiation
- when matter absorbs radiation, the energy of the radiation can cause either excitation or ionization
- ionization radiation more harmful than nonionization radiation
- most of energy of radiation absorbed by water molecules
- free radical – a substance with one ore more unpaired electrons
- can attack other biomolecules to produce more free radicals
- gamma rays most dangerous
- tissues that take most damage are the ones that reproduce at a rapid rate
- bone marrow, blood forming tissues, lymph nodes
21.9.1 Radiation Doses
-
- becquerel (Bq) – SI unit for activity of the radiation source; rate at which nuclear disintegrations are occurring
- 1 (Bq) = 1 nuclear disintegration/s
- curie (Ci) = 3.7x1010 disintegrations/s = rate of decay of 1g of radium
- two units used to measure amount of exposure to radiation: gray (Gy) and rad
- gray – SI unit of absorbed dose = absorption of 1 J of energy per kilogram of tissue
- rad (radiation absorbed dose) – absorption of 1x10-2 J of energy per kilogram of tissue
- 1 Gy = 100 rads
- relative biological effectiveness – RBE
- 1 for gamma and beta radiation, 10 for alpha radiation
- exact value varies with dose rate, total dose, and type of tissue affected
- rem (roentgen equivalent for man) – product of the radiation dose in rads and the RBE of the radiation gibes the effective dosage
- rem is unit of radiation damage that is usually used in medicine
- number of rems = (number of rads)(RBE)
- Sievert (Sv) – SI unit for dosage
- 1 Sv = 100 rem
- annual exposure = 360mrem
21.9.2 Radon
-
- radon exposure estimated to account for more than half annual exposure
- half-life of radon is 3.82 days
- decays into radioisotope polonium
- atoms of polonium can be trapped in lungs giving out alpha radiation causing lung cancer
- recommended levels of radon-222 in homes is to be less than 4 pCi per liter of air

