15.S: Chemical Equilibrium (Summary)
- Page ID
- 91278
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)chemical equilibrium – condition where the concentration of products and reactants do not change with time
15.1: The Concept of Equilibrium
- at equilibrium kf[A] = kr[B]
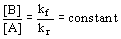
15.2: The Equilibrium Constant
- equilibrium condition can be reached from either forward or reverse direction
- Cato Maximillian Galdberg (1836-1902), and Peter Wauge (1833-1900)
- Law of mass action – relationship between concentrations of reactions and products at equilibrium
- If
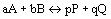 :
: 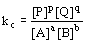 (equilibrium expression)
(equilibrium expression)- equilibrium expression depends only on stoichiometry of reaction and not mechanisms
- equilibrium constant:
- does not depend on initial concentrations
- does not matter if other substances present as long as they do not react with reactants or products
- varies with temperatures
- no units
15.2.1 Expressing Equilibrium Constants in Terms of Pressure, kp
15.2.2 The Magnitude of Equilibrium Constants
-
- k>>1; equilibrium lies to the right; products favored
- k<<1; equilibrium lies to the left; reactants favored
15.2.3 The Direction of the Chemical Equation and K
- equilibrium expression written in one direction is the reciprocal of the one in the other direction
15.4: Heterogeneous Equilibria
- homogeneous equilibria – substances in the same phase
- heterogeneous equilibria – substances in different phases
- concentration of pure liquid or solid
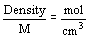
- density of pure liquid or solid is constant at any temperature
- if pure solid or liquid is involved in a reaction, its concentration is excluded from equilibrium expression
- pure solids must be present for equilibrium to be reached even through they are excluded from equilibrium expression
15.5: Calculating Equilibrium Constants
- determining unknown equilibrium concentrations
- 1) tabulate known initial and equilibrium concentrations
- 2) calculate change in concentration that occurs as system reaches equilibrium
-
- 3) use stoichiometry to determine change in concentration of unknown species
- 4) from initial concentrations and changes in concentrations, calculate equilibrium concentrations
15.4.1 Relating kc and kp
-
- PV = nRT; P = (n/V)RT = MRT
- PA = [A](RT)
- Kp=kc(RT)D n
- D n = change in moles from reactants to products
15.6: Applications of Equilibrium Constants
- equilibrium constant:
- 1) product direction reaction mixture will proceed
- 2) calculate concentrations of reactants and products once equilibrium is reached
15.5.1 Predicting the Direction of Reaction
-
- reaction quotient
- at equilibrium Q=k
- Q>k; reaction moves right to left
- Q<k; reaction moves left to right
15.5.2 Calculating of Equilibrium Concentrations
15.7: Le Chatelier's Principle
- if system at equilibrium is disturbed by change in temperature, pressure or concentration then system will shift equilibrium position
15.6.1 Change in Reactant or Product Concentration
-
- addition of substance will result in consummation of part of added substance
- if substance removed, reaction will move to produce more of the substance
15.6.2 Effects of Volume and Pressure Changes
-
- reducing volume, reaction shifts to reduce number of gas molecules
- increase volume, reaction shifts to produce more gas molecules
- increase pressure, decrease volume reduces total number of moles
- pressure volume changes do not affect k as long as temperature is constant
- changes concentrations of gaseous substances
15.6.3 Effect on Temperature Change
-
- endothermic: reactants + heat « products
- exothermic: reactants « products + heat
- increase temperature, equilibrium shifts in direction that absorbs heat
- endothermic: increase T, increase k
- exothermic: increase T, decrease k
- cooling shifts equilibrium to produce heat
15.6.4 The Effect of Catalysts
-
- catalysts increase rate at which equilibrium is obtained
- does not change composition of equilibrium mixture

