11.E: Properties of Reactions (Exercises)
- Page ID
- 59420
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)These are homework exercises to accompany Chapter 11 of the University of Kentucky's LibreText for CHE 103 - Chemistry for Allied Health.
Questions
(click here for solutions)
Q11.1.1
What is a free element, and what is the oxidation number for atoms that exist as a free element?
Q11.1.2
What is the highest oxidation number that sulfur can have? The lowest? Consider the number of valence electrons it has.
Q11.1.3
Determine the oxidation numbers of each of the atoms in the following.
- KMnO4
- OCl2
- H2C2O4
- Li3PO4
- NaClO
- Br2
- ClF3
- CaCl2
- K2O
Q11.1.4
Determine the oxidation number of each of the atoms in the following ions.
- NO2−
- NO3−
- Cr2O72−
- BrO3−
- ClO3−
- BO33−
- CO32−
- NH4+
- CrO42−
- S2O32−
(click here for solutions)
Q11.2.1
Explain why oxidation and reduction always occur together in a reaction.
Q11.2.2
What happens to the oxidizing agent in a redox reaction?
Q11.2.3
What happens to the reducing agent in a redox reaction?
Q11.2.4
Identify each process as an oxidation or a reduction.
- Rb → Rb+
- Te → Te2–
- 2H+ → H2
- P3– → P
- 2Cl– → Cl2
- Sn4+ → Sn2+
- Br2 → Br–
- Fe2+ → Fe3+
Q11.2.5
For each equation, 1) identify the oxidation numbers of each element, 2) determine if it is a redox reaction or not, and for redox reactions, 3) identify the species being oxidized and the species being reduced, and 4) identify the oxidizing and reducing agents.
- 2KClO3(s) → 2KCl(s) + 3O2(g)
- H2(g) + CuO(s) → Cu(s) + H2O(l)
- 2Al(s) + 3Mg(NO3)2(aq) → 2Al(NO3)3(aq) + 3Mg(s)
- 2HNO3(aq) + 6HI(aq) → 2NO(g) + 3I2(s) + 4H2O(l)
- AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
- 2FeCl3(aq) + H2S(g) → 2FeCl2(aq) + 2HCl(aq) + S(s)
Q11.2.6
Identify the change occurring with respect to the gain or loss of hydrogen or oxygen.
| a. | 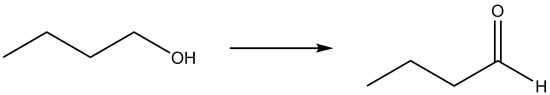 |
| b. |  |
| c. |  |
| d. |  |
| e. | 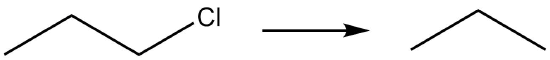 |
(click here for solutions)
Q11.3.1
Classify the following reactions as combination, decomposition, single replacement, double replacement, or combustion. For each, indicate if it is a redox reaction or not.
- Cd(s) + H2SO4(aq) → CdSO4(aq) + H2(g)
- 2Fe(s) + 3Cl2(g) → 2FeCl3(s)
- C7H8(l) + 9O2(g) → 7CO2(g) + 4H2O(g)
- 2NH4NO3(s) → 2N2(g) + O2(g) + 4H2O(g)
- 2CoCl3(aq) + 3Pb(NO3)2(aq) → 2Co(NO3)3(aq) + 3PbCl2(s)
Q11.3.2
Balance the following equations and classify the following reactions as combination, decomposition, single replacement, double replacement, or combustion. For each, indicate if it is a redox reaction or not.
- Na + Cl2 → NaCl
- Na3PO4 + KOH → NaOH + K3PO4
- P4 + O2 → P2O3
- N2 + H2 → NH3
- Al + HCl → H2 + AlCl3
- H2O2 → H2O + O2
- NH3 + CuO → Cu + N2 + H2O
- NH4NO3 → N2O + H2O
Q11.3.3
What do the products of combustion reactions have in common?
Q11.3.4
Write and balance combustion reactions for the following compounds.
- methane (CH4)
- propane (C3H8)
- octane (C8H18)
- ethanol (CH3CH2OH)
- sucrose (C12H22O11)
(click here for solutions)
Q11.4.1
In a certain reaction, the energy of the reactants is less than the energy of the products (reaction consumes energy). Is the reaction endothermic or exothermic?
Q11.4.2
What are the two driving forces for all chemical reactions and physical processes?
Q11.4.3
Does entropy determine the spontaneity of a reaction? Does enthalpy determine the spontaneity of a reaction?
Q11.4.4
How does an increase in temperature affect the entropy of a system?
Q11.4.5
Which system has the greater entropy in each of the following?
- solid sodium chloride or a sodium chloride solution
- bromine liquid or bromine vapor
- 25 g of water at 80°C or 25 g of water at 50°C
- liquid mercury or solid mercury
Q11.4.6
How does the entropy of a system change for each of the following processes?
- A solid melts.
- A liquid freezes.
- A liquid boils.
- A vapor condenses to a liquid.
- Sugar dissolves in water.
- A solid sublimes.
(click here for solutions)
Q11.5.1
What is true about the relative amounts of reactants and products at the end of a spontaneous reaction?
Q11.5.2
Can a proposed reaction be spontaneous and yet still not be observed to occur? Explain.
Q11.5.3
The forward reaction is spontaneous for a particular reversible reaction. What can you conclude about the spontaneity of the reverse reaction?
Q11.5.4
Explain how free energy is used to determine whether or not a reaction is spontaneous.
Q11.5.5
Under what conditions of enthalpy and entropy change is a reaction always spontaneous? Under what conditions is a reaction never spontaneous?
Q11.5.6
If the enthalpy change is unfavorable (endothermic), but the entropy change is favorable (increasing), would a high temperature or a low temperature be more likely to lead to a spontaneous reaction?
Q11.5.7
If the enthalpy change is favorable and the entropy change is favorable, would the reaction be spontaneous at high temperatures, low temperatures, or all temperatures?
(click here for solutions)
Q11.6.1
In what unit is the rate of a chemical reaction typically expressed?
Q11.6.2
A 2.50 M solution undergoes a chemical reaction. After 3.00 minutes, the concentration of the solution is 2.15 M. What is the rate of change in M/s?
Q11.6.3
Substance A disappears at a rate of 0.0250 M/s. If the initial concentration is 4.00 M, what is the concentration after one minute?
Q11.6.4
The concentration of product B increases from 0 to 1.75 M in 45 seconds. What is the rate of formation of B?
Q11.6.5
The concentration of product B increases from 0.50 M to 1.25 M in 2.5 seconds. What is the rate of formation of B?
Q11.6.6
Reactant B goes from 2.25 M to 1.50 M in 0.85 seconds. What is the rate of change of B?
Q11.6.7
Does every collision between reacting particles lead to the formation of products? Explain.
Q11.6.8
What two conditions must be met in order for a collision to be effective?
Q11.6.9
Explain why the activation energy of a reaction is sometimes referred to as a barrier.
Q11.6.10
Why is it difficult to study activated complexes?
Q11.6.11
Explain how reaction rates can be affected by
- changes in concentration.
- changes in pressure.
- increased surface area.
- changes in temperatures.
Q11.6.12
What is the effect of a catalyst on the rate of a reaction?
Q11.6.13
Explain how the presence of a catalyst affects the activation energy of a reaction.
Q11.6.14
Zinc metal reacts with hydrochloric acid. Which one would result in the highest rate of reaction?
- A solid piece of zinc in 1 M HCl
- A solid piece of zinc in 3 M HCl
- Zinc powder in 1 M HCl
- Zinc powder in 3 M HCl
Q11.6.15
Use the potential energy diagram below to answer the following questions.
- What is the potential energy of the reactants?
- What is the potential energy of the products?
- What is the heat of reaction (ΔH = Eproducts − Ereactants)?
- What is the potential energy of the activated complex?
- What is the activation energy for the reaction?
- Is the reaction endothermic or exothermic?
- Which of the values in a-e above would be changed by the use of a catalyst in the reaction?
- What is the activation energy of the reverse reaction?
- What is the heat of reaction (ΔH = Eproducts − Ereactants) of the reverse reaction?
Q11.6.16
Use the potential energy diagram below to answer the following questions.
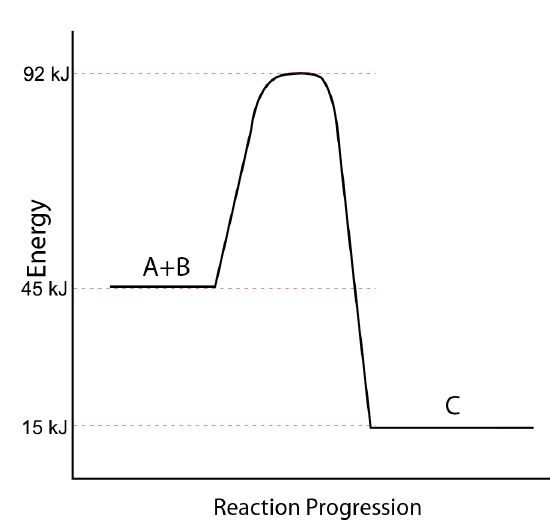
- What is the potential energy of the reactants?
- What is the potential energy of the products?
- What is the heat of reaction (ΔH = Eproducts − Ereactants)?
- What is the potential energy of the activated complex?
- What is the activation energy for the reaction?
- Is the reaction endothermic or exothermic?
- Which of the values in a-e above would be changed by the use of a catalyst in the reaction?
- What is the activation energy of the reverse reaction?
- What is the heat of reaction (ΔH = Eproducts − Ereactants) of the reverse reaction?
Answers
11.1: Oxidation Numbers
Q11.1.1
Any element by itself, either monatomic or diatomic, without a charge. The oxidation number of any free element is zero.
Q11.1.2
Sulfur can have an oxidation number up to +6 or as low as \(-2\).
Q11.1.3
- \(\overset{+1}{\text{K}}\overset{+7}{\text{Mn}}\overset{-2}{\text{O}_4}\)
- Rule 2: K forms an ion with a 1+ charge so its oxidation number is +1.
- Rule 3: O has an oxidation number of \(-2\).
- Rule 6 for Mn: \(1+x+4(-2)=0\\
1+x+-8=0\\
x-7=0\\
x=7\)
- \(\overset{-2}{\text{O}}\overset{+1}{\text{Cl}_2}\)
- Rule 3: O has an oxidation number of \(-2\).
- Rule 6 for Cl: \(-2+2x=0\\
2x=-2\\
x=1\)
- \(\overset{+1}{\text{H}_2}\overset{+3}{\text{C}_2}\overset{-2}{\text{O}_4}\)
- Rule 3: O has an oxidation number of \(-2\).
- Rule 4: H has an oxidation number of \{+1\).
- Rule 6 for C: \(2(+1)+2x+4(-2)=0\\
2+2x-8=0\\
2x-6=0\\ 2x=6\\x=3\)
- \(\overset{+1}{\text{Li}_3}\overset{+5}{\text{P}}\overset{-2}{\text{O}_4}\)
- Rule 2: Li forms an ion with a 1+ charge so its oxidation number is +1.
- Rule 3: O has an oxidation number of \(-2\).
- Rule 6 for P: \(3(+1)+x+4(-2)=0\\
3+x+-8=0\\
x-5=0\\
x=5\)
- \(\overset{+1}{\text{Na}}\overset{+1}{\text{Cl}}\overset{-2}{\text{O}}\)
- Rule 2: Na forms an ion with a 1+ charge so its oxidation number is +1.
- Rule 3: O has an oxidation number of \(-2\).
- Rule 6 for Cl: \(1+x+-2=0\\
x-1=0\\
x=1\)
- \(\overset{0}{\text{Br}_2}\)
- Rule 1: Oxidation number of a free element is zero.
- \(\overset{+3}{\text{Cl}} \overset{-1}{\text{F}_3}\)
- Rule 5: F has an oxidation number of \(-1\).
- Rule 6 for Cl: \(x+3(-1)=0\\ x-3=0\\ x=3\)
- \(\overset{+2}{\text{Ca}}\overset{-1}{\text{Cl}_2}\)
- Rule 2: Ca forms an ion with a 2+ charge so its oxidation number is +2.
- Rule 6 for Cl: \(2+2(x)=0\\
2x=-2\\
x=-1\)
- \(\overset{+1}{\text{K}_2}\overset{-2}{\text{O}}\)
- Rule 2: K forms an ion with a 1+ charge so its oxidation number is +1.
- Rule 3: O has an oxidation number of \(-2\).
Q11.1.4
Determine the oxidation number of each of the atoms in the following ions.
- \(\overset{+3}{\text{N}}\overset{-2}{\text{O}^-_2}\)
- Rule 2: O has an oxidation number of \(-2\).
- Rule 7 for N: \(x+2(-2)=-1\\
x-4=-1\\
x=3\)
- \(\overset{+5}{\text{N}}\overset{-2}{\text{O}^-_3}\)
- Rule 2: O has an oxidation number of \(-2\).
- Rule 7 for N: \(x+3(-2)=-1\\
x-6=-1\\
x=5\)
- \(\overset{+6}{\text{Cr}_2}\overset{-2}{\text{O}^{2-}_7}\)
- Rule 2: O has an oxidation number of \(-2\).
- Rule 7 for Cr: \(2x+7(-2)=-2\\
2x-14=-2\\
2x=12\\
x=6\)
- \(\overset{+5}{\text{Br}}\overset{-2}{\text{O}^-_3}\)
- Rule 2: O has an oxidation number of \(-2\).
- Rule 7 for Br: \(x+3(-2)=-1\\
x-6=-1\\
x=5\)
- \(\overset{+5}{\text{Cl}}\overset{-2}{\text{O}^-_3}\)
- Rule 2: O has an oxidation number of \(-2\).
- Rule 7 for Cl: \(x+3(-2)=-1\\
x-6=-1\\
x=5\)
- \(\overset{+3}{\text{B}}\overset{-2}{\text{O}^-_3}\)
- Rule 2: O has an oxidation number of \(-2\).
- Rule 7 for B: \(x+3(-2)=-3\\
x-6=-3\\
x=3\)
- \(\overset{+4}{\text{C}}\overset{-2}{\text{O}^{2-}_3}\)
- Rule 2: O has an oxidation number of \(-2\).
- Rule 7 for C: \(x+3(-2)=-2\\
x-6=-2\\
x=4\)
- \(\overset{-3}{\text{N}}\overset{+1}{\text{H}^+_4}\)
- Rule 2: H has an oxidation number of \(+1\).
- Rule 7 for N: \(x+4(+1)=+1\\
x+4=1\\
x=-3\)
- \(\overset{+6}{\text{Cr}}\overset{-2}{\text{O}^{2-}_4}\)
- Rule 2: O has an oxidation number of \(-2\).
- Rule 7 for Cr: \(x+4(-2)=-2\\
x-8=-2\\
x=6\)
- \(\overset{+2}{\text{S}_2}\overset{-2}{\text{O}^{2-}_3}\)
- Rule 2: O has an oxidation number of \(-2\).
- Rule 7 for S: \(2x+3(-2)=-2\\
2x-6=-2\\
2x=4\\
x=2\)
11.2: The Nature of Oxidation and Reduction
Q11.2.1
Oxidation and reduction are opposite processes. Electrons are lost by one substance and gained by another so they have a transfer of electrons.
Q11.2.2
The oxidizing agent is reduced.
Q11.2.3
The reducing agent is oxidized.
Q11.2.4
- oxidation
- reduction
- reduction
- oxidation
- oxidation
- reduction
- reduction
- oxidation
Q11.2.5
- 2KClO3(s) → 2KCl(s) + 3O2(g)
- \(\overset{+1}{2\text{K}}\overset{+5}{\text{Cl}}\overset{-2}{\text{O}_3}\rightarrow \overset{+1}{2\text{K}}\overset{-1}{\text{Cl}}~+~\overset{0}{3\text{O}_2}\)
- redox
- oxygen is being oxidized, chlorine is being reduced
- KClO3 is the oxidizing and reducing agent (specifically, the oxygen is the reducing agent and the chlorine is the oxidizing agent)
- H2(g) + CuO(s) → Cu(s) + H2O(l)
- \(\overset{0}{\text{H}_2}~+~\overset{+2}{\text{Cu}}\overset{-2}{\text{O}}\rightarrow \overset{0}{\text{Cu}}~+~\overset{+1}{\text{H}_2}\overset{-2}{\text{O}}\)
- redox
- hydrogen is being oxidized, copper is being reduced
- CuO is the oxidizing agent, H2 is the reducing agent
- 2Al(s) + 3Mg(NO3)2(aq) → 2Al(NO3)3(aq) + 3Mg(s)
- \(overset{0}{2\text{Al}}~+~\overset{+2}{3\text{Mg}}\overset{+5} {\text{(N}}\overset{-2}{\text{O}_3\text{)}_2} \rightarrow \overset{+3}{2\text{Al}}\overset{+5}
{\text{(N}}\overset{-2}{\text{O}_3\text{)}_3}~+~\overset{0}{3\text{Mg}}\) - redox
- aluminum is being oxidized, magnesium is being reduced
- Mg(NO3)2 is the oxidizing agent, Al is the reducing agent
- \(overset{0}{2\text{Al}}~+~\overset{+2}{3\text{Mg}}\overset{+5} {\text{(N}}\overset{-2}{\text{O}_3\text{)}_2} \rightarrow \overset{+3}{2\text{Al}}\overset{+5}
- 2HNO3(aq) + 6HI(aq) → 2NO(g) + 3I2(s) + 4H2O(l)
- \(\overset{+1}{2\text{H}}\overset{+5}{\text{N}}\overset{-2}{\text{O}_3}~+~\overset{+1}{6\text{H}}\overset{-1}{\text{I}} \rightarrow \overset{+2}{2\text{N}}\overset{-2}{\text{O}}~+~\overset{0} {3\text{I}_2}~+~\overset{+1}{4\text{H}_2}\overset{-2}{\text{O}}\)
- redox
- iodine is being oxidized, nitrogen is being reduced
- HNO3 is the oxidizing agent, HI is the reducing agent
- AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
- \(\overset{+1}{\text{Ag}}\overset{+5}{\text{N}}\overset{-2}{\text{O}_3}~+~\overset{+1}{\text{Na}}\overset{-1}{\text{Cl}} \rightarrow \overset{+1}{\text{Ag}}\overset{-1}{\text{Cl}}~+~\overset{+1}{\text{Na}}\overset{+5}{\text{N}}\overset{-2}{\text{O}_3}\)
- not redox
- 2FeCl3(aq) + H2S(g) → 2FeCl2(aq) + 2HCl(aq) + S(s)
- \(\overset{+3}{2\text{Fe}}\overset{-1}{\text{Cl}_3}~+~\overset{+1}{\text{H}_2}\overset{-2}{\text{S}} \rightarrow \overset{+2}{2\text{Fe}}\overset{-1}{\text{Cl}_2}~+~\overset{+1}{2\text{H}}\overset{-1}{\text{Cl}}~+~\overset{0}{\text{S}}\)
- redox
- sulfur is being oxidized, iron is being reduced
- FeCl3 is the oxidizing agent, H2S is the reducing agent
Q11.2.6
- Oxidation due to loss of hydrogen.
- Reduction due to loss of oxygen.
- Reduction due to loss of oxygen or gain of hydrogen.
- Reduction due to gain of hydrogen.
- Reduction due to gain of hydrogen.
11.3: Types of Chemical Reactions
Q11.3.1
- single replacement (single displacement), redox
- combination (synthesis), redox
- combustion, redox
- decomposition, redox
- double replacement (double displacement), not redox
Q11.3.2
- 2Na + Cl2 → 2NaCl, combination (synthesis), redox
- Na3PO4 + 3KOH → 3NaOH + K3PO4, double replacement (double displacement), not redox
- P4 + 3O2 → 2P2O3, combination (synthesis), redox
- N2 + 3H2 → 2NH3, combination (synthesis), redox
- 2Al + 6HCl → 3H2 + 2AlCl3, single replacement (single displacement), redox
- 2H2O2 → 2H2O + O2, decomposition, redox
- 2NH3 + 3CuO → 3Cu + N2 + 3H2O, decomposition, redox
- NH4NO3 → N2O + 2H2O, decomposition, not redox
Q11.3.3
Combustion produces CO2 and H2O.
Q11.3.4
- CH4 + 2O2 → CO2 + 2H2O
- C3H8 + 5O2 → 3CO2 + 4H2O
- 2C8H18 + 25O2 → 16CO2 + 18H2O
- CH3CH2OH + 3O2 → 2CO2 + 3H2O
- C12H22O11 + 12O2 → 12CO2 + 11H2O
11.4: Entropy
Q11.4.1
endothermic
Q11.4.2
enthalpy and entropy
Q11.4.3
Neither entropy nor enthalpy determine the spontaneity of a reaction but both contribute to determining the spontaneity.
Q11.4.4
Entropy increases with temperature.
Q11.4.5
Which system has the greater entropy in each of the following?
- solution
- vapor
- 80°C
- liquid
Q11.4.6
How does the entropy of a system change for each of the following processes?
- increases
- decreases
- increasese
- decreases
- increases
- increases
11.5: Spontaneous Reactions and Free Energy
Q11.5.1
There are more products than reactants.
Q11.5.2
Yes, because it is a very slow reaction.
Q11.5.3
The reverse reaction is not spontaneous.
Q11.5.4
Free energy (\(\Delta G\)) is negative for spontaneous reactions and positive for non-spontaneous reactions.
Q11.5.5
\(\Delta G\) is negative (spontaneous) at all temperatures when \(\Delta H\) is negative and \(\Delta S\) is positive.
\(\Delta G\) is positive (non-spontaneous) at all temperatures when \(\Delta H\) is positive and \(\Delta S\) is negative.
Q11.5.6
high temperature
Q11.5.7
all temperatures
11.6: Rates of Reactions
Q11.6.1
molarity per second, \(\frac{M}{s}\) (may be written as \(Ms^{-1}\))
Q11.6.2
\(rate=\frac{\Delta [A]}{\Delta t}\\=\frac{2.15-2.50\;M}{180. \;s}\\ =\frac{-0.35\;M}{180.\;s}\\ =-0.0019\;\frac{M}{s}\)
Q11.6.3
\(60\;s\left(\frac{0.0250\;M}{s}\right)=1.50\;M\;\text{lost}\\
initial + change =final\\
4.00\;M+(-1.50\;M)=2.50\;M\)
Q11.6.4
\(rate=\frac{\Delta [B]}{\Delta t}\\=\frac{1.75-0\;M}{45 \;s}\\ =0.039\;\frac{M}{s}\)
Q11.6.5
\(rate=\frac{\Delta [B]}{\Delta t}\\=\frac{1.25-0.50\;M}{2.5 \;s}\\ =\frac{0.75\;M}{2.5\;s}\\ =0.30\;\frac{M}{s}\)
Q11.6.6
\(rate=\frac{\Delta [B]}{\Delta t}\\=\frac{1.50-2.25\;M}{0.85 \;s}\\ =\frac{-0.75\;M}{0.85\;s}\\ =-0.88\;\frac{M}{s}\)
Q11.6.7
No, because collisions have to occur at the correct orientation with sufficient energy.
Q11.6.8
Correct orientation and sufficient energy.
Q11.6.9
Because it is the energy requirement that must be met before the reaction can occur. When there is not enough energy, the reaction is blocked from occurring.
Q11.6.10
They are very short-lived.
Q11.6.11
- Increasing concentration increases the probability of an effective collision so the reaction rate will increase. The reverse is true for a decrease in concentration.
- Change in pressure of a substance involved in the reaction will have the same effect as a change in concentration.
- Increasing the surface area creates more places for reactants to interact which will increase the probability of an effective collision so the reaction rate will increase.
- Increasing the temperature will increase the energy of collisions so a greater number of collisions will have sufficient energy to overcome the activation barrier which will result in an increase in the reaction rate. Decreasing the temperature will have the reverse effect.
Q11.6.12
Catalysts increase the rate of a reaction by lowering the activation energy.
Q11.6.13
Catalysts provide an alternate mechanism or "path" for the reaction to occur. This new mechanism has a lower activation energy so more collisions have enough energy to overcome the barrier which will increase the reaction rate.
Q11.6.14
d. The powder has a higher surface area than a solid piece of zinc and the higher concentration will result in a higher reaction rate.
Q11.6.15
- 20 kJ/mol
- 50 kJ/mol
- 30 kJ/mol
- 100 kJ/mol
- 80 kJ/mol
- endothermic
- d (energy of the activated complex) and e (activation energy)
- 50 kJ/mol
- \(-\)30 kJ/mol
Q11.6.16
- 45 kJ/mol
- 15 kJ/mol
- \(-\)30 kJ/mol
- 92 kJ/mol
- 47 kJ/mol
- exothermic
- d (energy of the activated complex) and e (activation energy)
- 77 kJ/mol
- 30 kJ/mol

