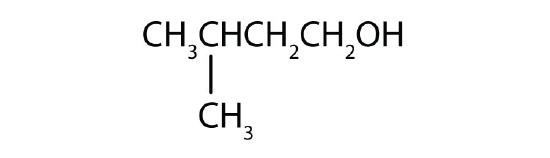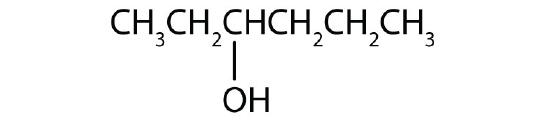14.2: Alcohols - Nomenclature and Classification
- Page ID
- 133461
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Identify the general structure for an alcohol.
- Identify the structural feature that classifies alcohols as primary, secondary, or tertiary.
- Name alcohols with both common names and IUPAC names
An alcohol is an organic compound with a hydroxyl (OH) functional group on an aliphatic carbon atom. Because OH is the functional group of all alcohols, we often represent alcohols by the general formula ROH, where R is an alkyl group. Alcohols are common in nature. Most people are familiar with ethyl alcohol (ethanol), the active ingredient in alcoholic beverages, but this compound is only one of a family of organic compounds known as alcohols. The family also includes such familiar substances as cholesterol and the carbohydrates. Methanol (CH3OH) and ethanol (CH3CH2OH) are the first two members of the homologous series of alcohols.
Nomenclature of Alcohols
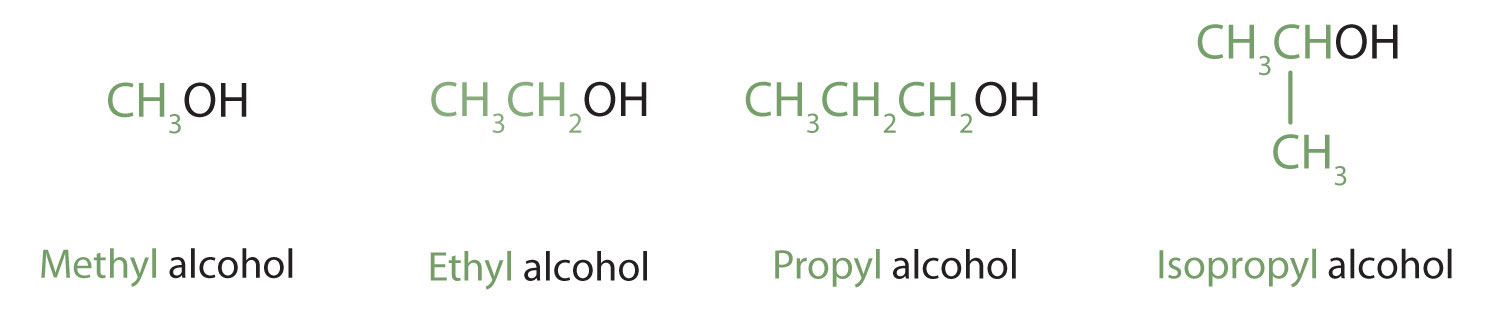
Alcohols with one to four carbon atoms are frequently called by common names, in which the name of the alkyl group is followed by the word alcohol:
According to the International Union of Pure and Applied Chemistry (IUPAC), alcohols are named by changing the ending of the parent alkane name to -ol. Here are some basic IUPAC rules for naming alcohols:
- The longest continuous chain (LCC) of carbon atoms containing the OH group is taken as the parent compound—an alkane with the same number of carbon atoms. The chain is numbered from the end nearest the OH group.
- The number that indicates the position of the OH group is prefixed to the name of the parent hydrocarbon, and the -e ending of the parent alkane is replaced by the suffix -ol. (In cyclic alcohols, the carbon atom bearing the OH group is designated C1, but the 1 is not used in the name.) Substituents are named and numbered as in alkanes.
- If more than one OH group appears in the same molecule (polyhydroxy alcohols), suffixes such as -diol and -triol are used. In these cases, the -e ending of the parent alkane is retained.
Figure \(\PageIndex{1}\) shows some examples of the application of these rules.
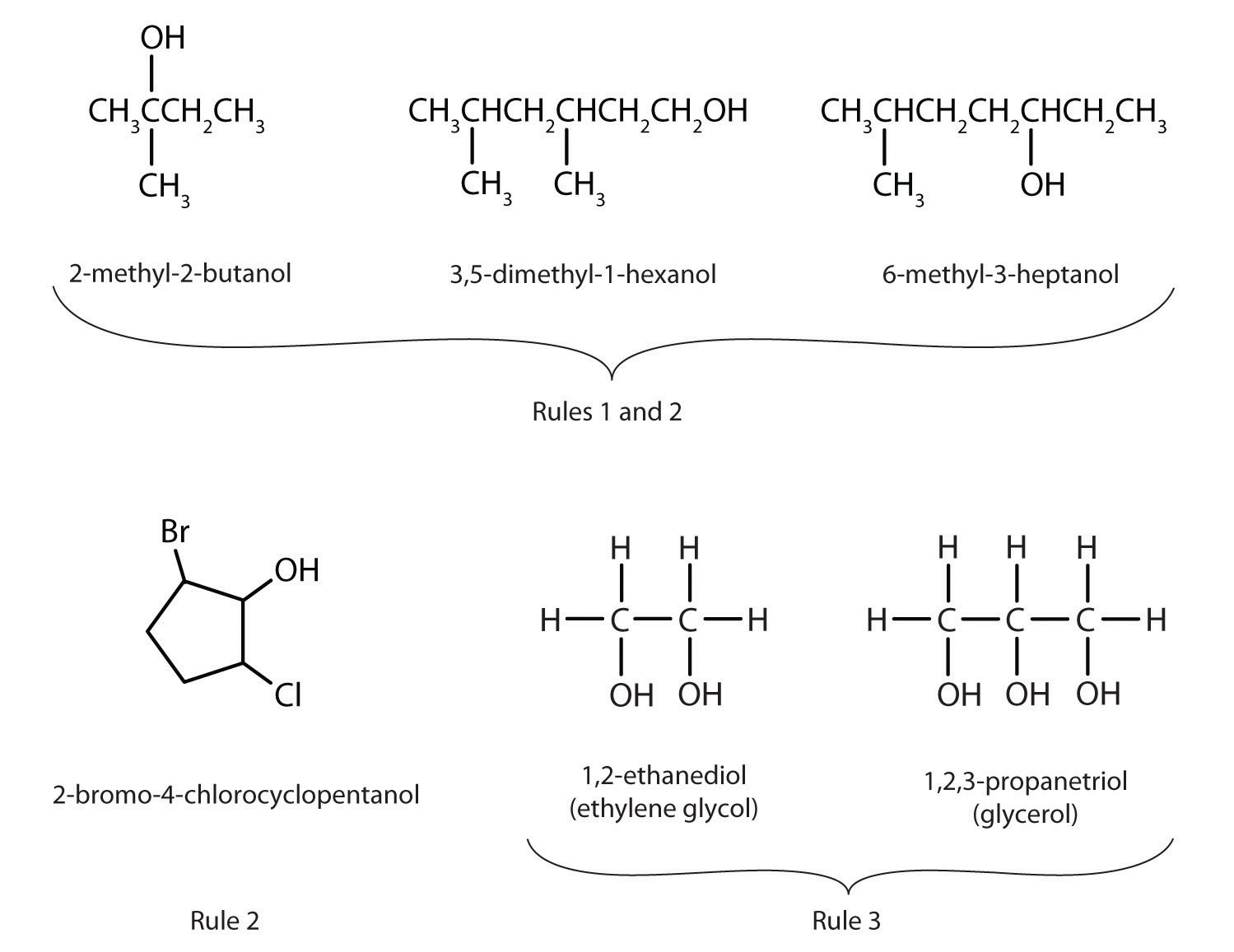
Figure \(\PageIndex{1}\): IUPAC Rules for Alcohols. The names and structures of some alcohols demonstrate the use of IUPAC rules.
Example \(\PageIndex{1}\)
Give the IUPAC name for each compound.
-
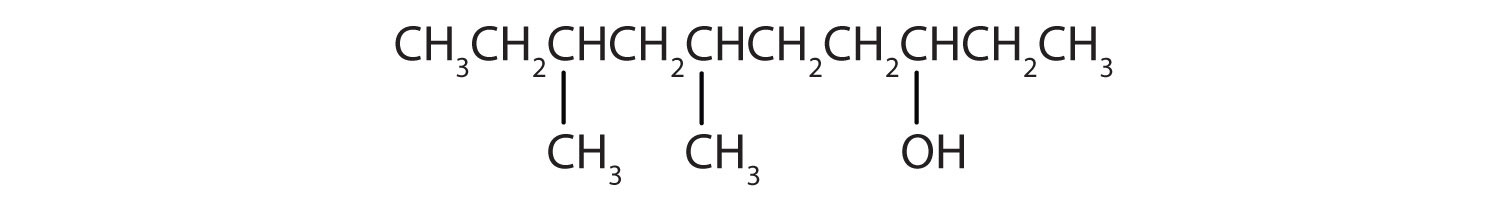
- HOCH2CH2CH2CH2CH2OH
SOLUTION
-
Ten carbon atoms in the LCC makes the compound a derivative of decane (rule 1), and the OH on the third carbon atom makes it a 3-decanol (rule 2).
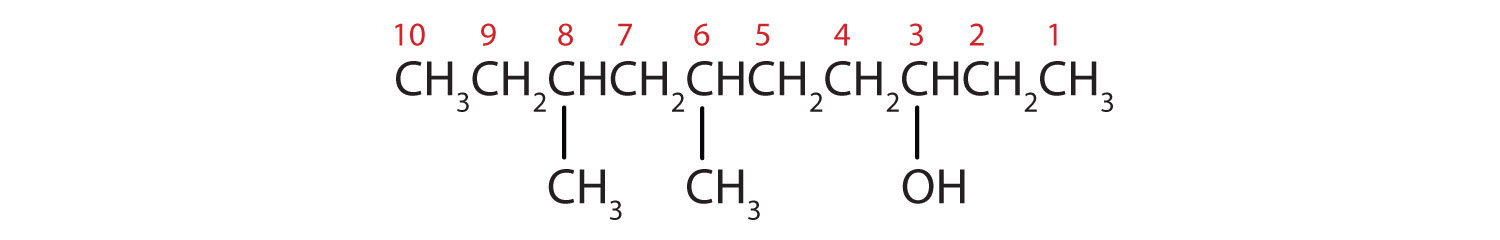
The carbon atoms are numbered from the end closest to the OH group. That fixes the two methyl (CH3) groups at the sixth and eighth positions. The name is 6,8-dimethyl-3-decanol (not 3,5-dimethyl-8-decanol).
-
Five carbon atoms in the LCC make the compound a derivative of pentane. Two OH groups on the first and fifth carbon atoms make the compound a diol and give the name 1,5-pentanediol (rule 3).
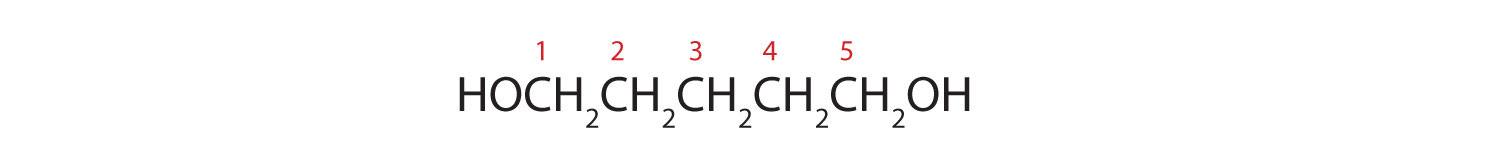
Exercise \(\PageIndex{1}\)
Give the IUPAC name for each compound.
Example \(\PageIndex{2}\)
Draw the structure for each compound.
- 2-hexanol
- 3-methyl-2-pentanol
SOLUTION
-
The ending -ol indicates an alcohol (the OH functional group), and the hex- stem tells us that there are six carbon atoms in the LCC. We start by drawing a chain of six carbon atoms: –C–C–C–C–C–C–.
The 2 indicates that the OH group is attached to the second carbon atom.
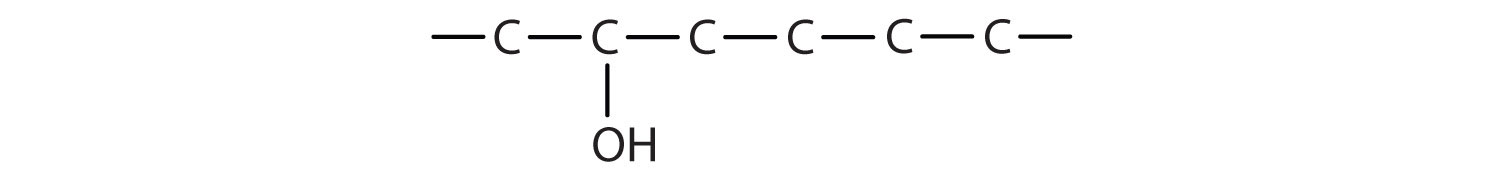
Finally, we add enough hydrogen atoms to give each carbon atom four bonds.
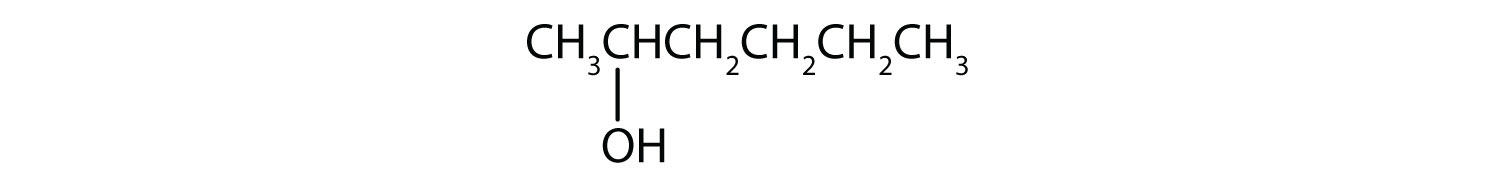
-
The ending -ol indicates an OH functional group, and the pent- stem tells us that there are five carbon atoms in the LCC. We start by drawing a chain of five carbon atoms:
–C–C–C–C–C–The numbers indicate that there is a methyl (CH3) group on the third carbon atom and an OH group on the second carbon atom.
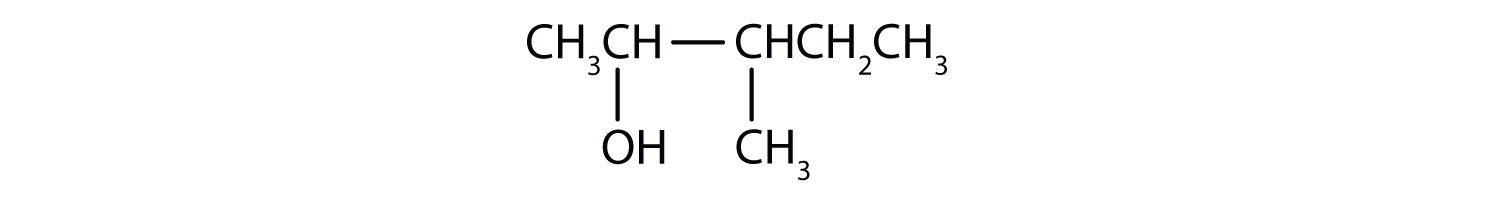
Exercise \(\PageIndex{2}\)
Draw the structure for each compound.
-
3-heptanol
-
2-methyl-3-hexanol
Classification of Alcohols
Some of the properties of alcohols depend on the number of carbon atoms attached to the specific carbon atom that is attached to the OH group. Alcohols can be grouped into three classes on this basis.
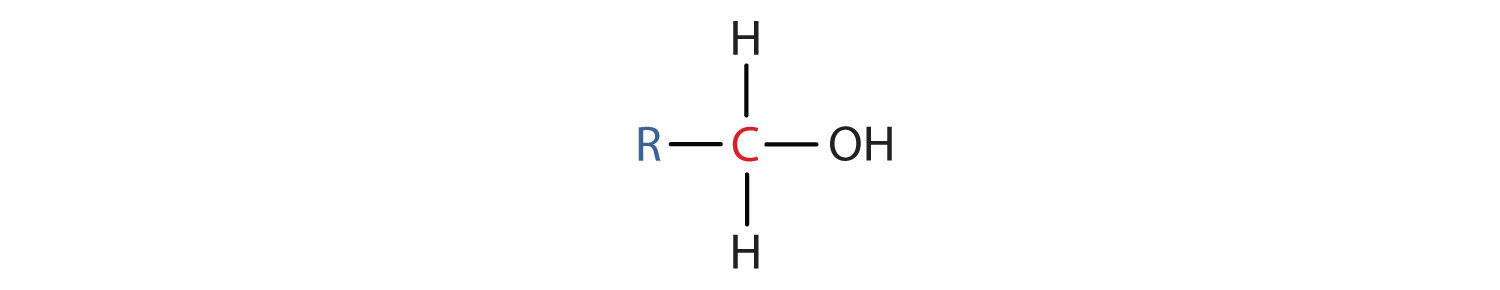
- A primary (1°) alcohol is one in which the carbon atom (in red) with the OH group is attached to one other carbon atom (in blue). Its general formula is RCH2OH.
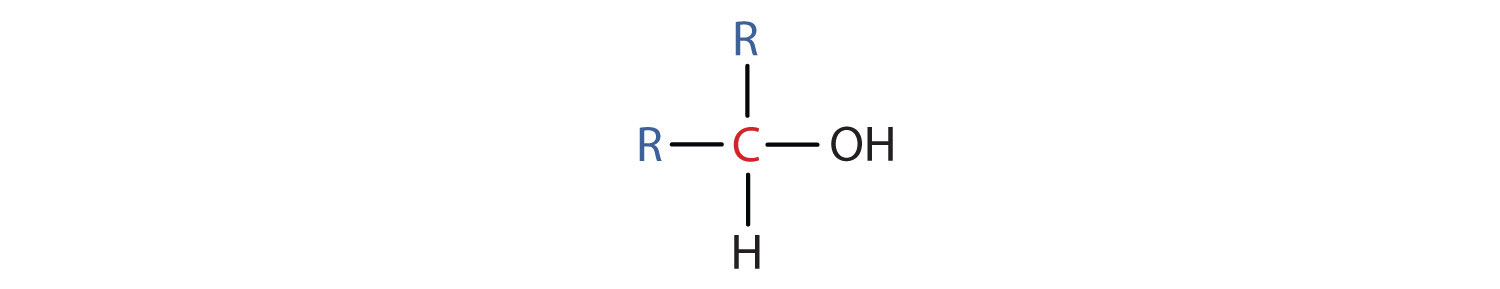
- A secondary (2°) alcohol is one in which the carbon atom (in red) with the OH group is attached to two other carbon atoms (in blue). Its general formula is R2CHOH.
- A tertiary (3°) alcohol is one in which the carbon atom (in red) with the OH group is attached to three other carbon atoms (in blue). Its general formula is R3COH.
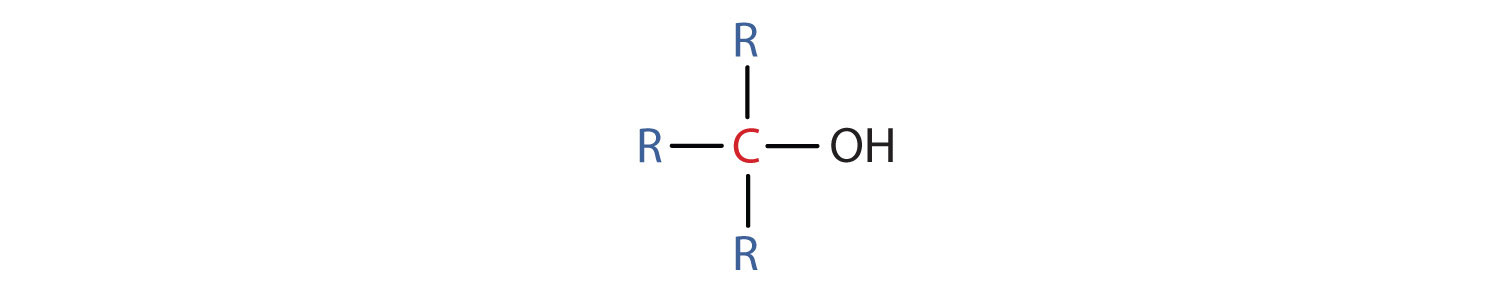
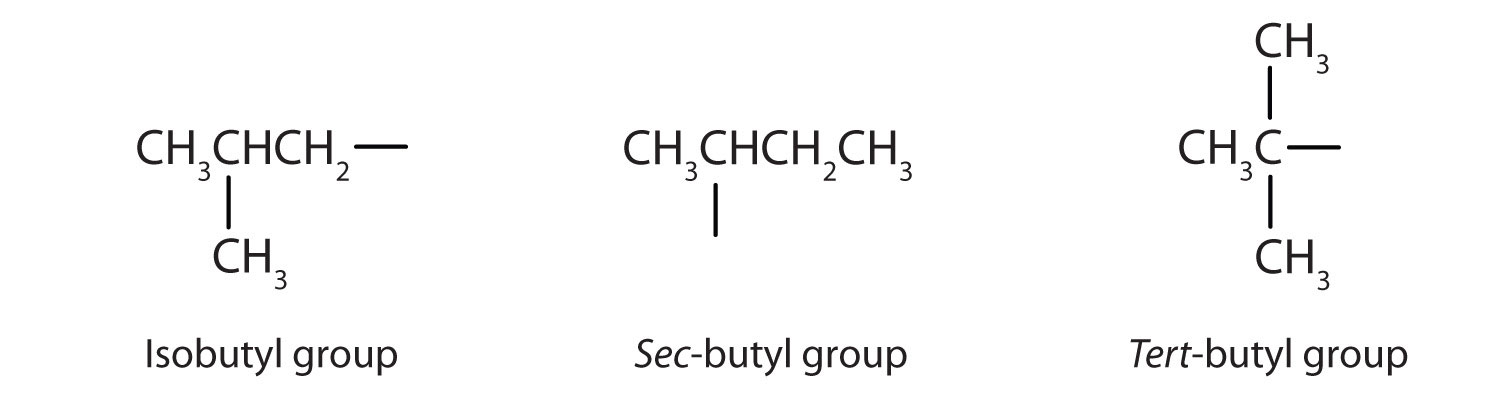
Table \(\PageIndex{1}\) names and classifies some of the simpler alcohols. Some of the common names reflect a compound’s classification as secondary (sec-) or tertiary (tert-). These designations are not used in the IUPAC nomenclature system for alcohols. Note that there are four butyl alcohols in the table, corresponding to the four butyl groups: the butyl group (CH3CH2CH2CH2) discussed before, and three others:
| Condensed Structural Formula | Class of Alcohol | Common Name | IUPAC Name |
|---|---|---|---|
| CH3OH | — | methyl alcohol | methanol |
| CH3CH2OH | primary | ethyl alcohol | ethanol |
| CH3CH2CH2OH | primary | propyl alcohol | 1-propanol |
| (CH3)2CHOH | secondary | isopropyl alcohol | 2-propanol |
| CH3CH2CH2CH2OH | primary | butyl alcohol | 1-butanol |
| CH3CH2CHOHCH3 | secondary | sec-butyl alcohol | 2-butanol |
| (CH3)2(CH3)2CHCH2OH | primary | isobutyl alcohol | 2-methyl-1-propanol |
| (CH3)3COH | tertiary | tert-butyl alcohol | 2-methyl-2-propanol |
 |
secondary | cyclohexyl alcohol | cyclohexanol |
Summary
In the IUPAC system, alcohols are named by changing the ending of the parent alkane name to -ol. Alcohols are classified according to the number of carbon atoms attached to the carbon atom that is attached to the OH group.
Concept Review Exercises
-
Is isobutyl alcohol primary, secondary, or tertiary? Explain.
-
What is the LCC in 2-ethyl-1-hexanol? What is taken as the LCC in naming the compound? Explain.
Answers
-
primary; the carbon atom bearing the OH group is attached to only one other carbon atom
-
7 carbon atoms; the 6-atom chain includes the carbon atom bearing the OH group
Exercises
-
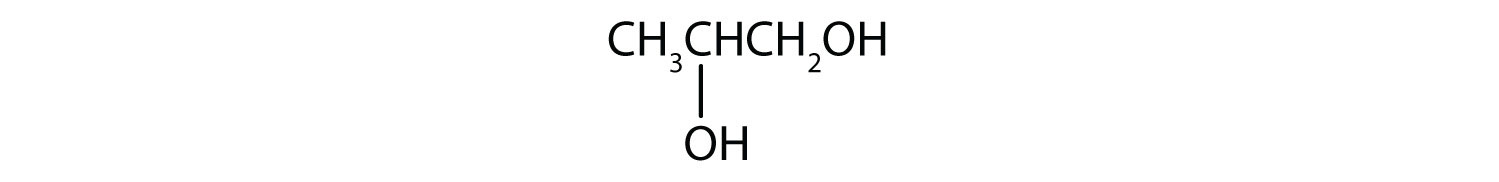
Name each alcohol and classify it as primary, secondary, or tertiary.
- CH3CH2CH2CH2CH2CH2OH
-
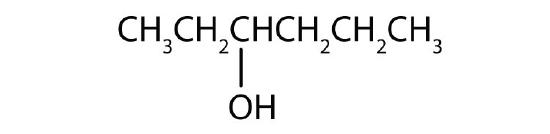
-
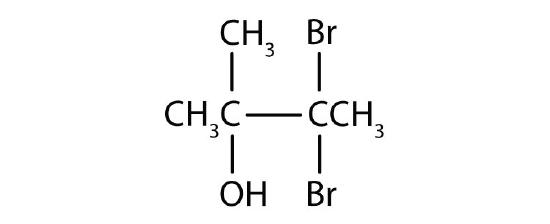
-
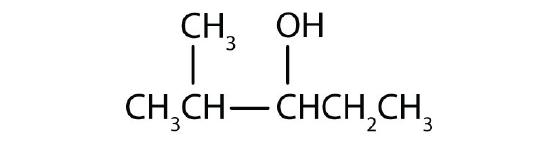
Name each alcohol and classify it as primary, secondary, or tertiary.
-
-
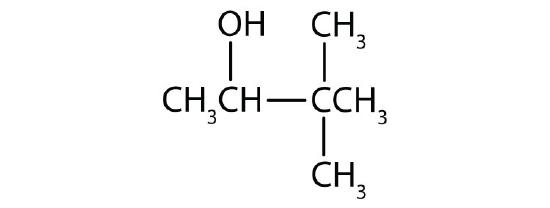
Draw the structure for each alcohol.
- 3-hexanol
- 3,3-dimethyl-2-butanol
- cyclobutanol
-
Draw the structure for each alcohol.
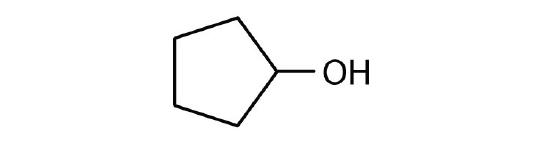
- cyclopentanol
- 4-methyl-2-hexanol
- 4,5-dimethyl-3-heptanol
Answers
-
- 1-hexanol; primary
- 3-hexanol; secondary
- 3,3-dibromo-2-methyl-2-butanol; tertiary