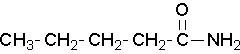22.3 Nomenclature of Acid Derivatives
- Page ID
- 28413
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
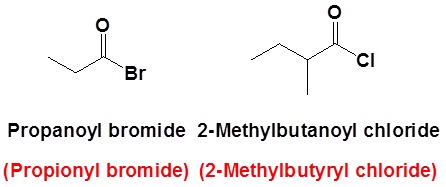
Nomenclature of acid halides
The nomenclature of acid halides starts with the name of the corresponding carboxylic acid. The –ic acid ending is removed and replaced with the ending -yl followed by the name of the halogen with an –ide ending. This is true for both common and IUPAC nomenclature. The carbonyl carbon is given the #1 location number. It is not necessary to include the location number in the name because it is assumed that the functional group will be on the end of the parent chain.
| Example 1: |
|---|
|
|
The acid anhydride functional group results when two carboxylic acids combine and lose water (anhydride = without water). Symmetrical acid anhydrides are named like carboxylic acids except the ending -acid is replaced with -anhydride. This is true for both the IUPAC and Common nomenclature.
Nomenclature of Anhydrides
Symmetrical anhydrides
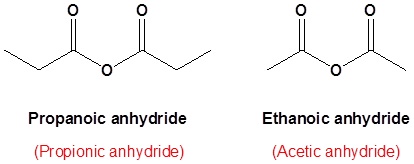
Unsymmetrical acid anhydrides
Unsymmetrical acid anhydrides are named by first naming each component carboxylic acid alphabetically arranged (without the word acid) followed by spaces and then the word anhydride.
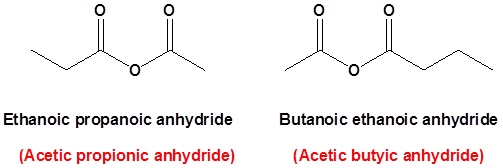
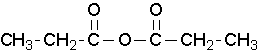
propanoic anhydride
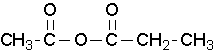
ethanoic propanoic anhydride
Try to name the following compound
Try to draw a structure for the following compound�
- 1,2-benzenedicarboxylic anhydride J
Common names that you should know
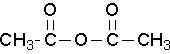
acetic anhydride (Try to name this anhydride by the proper name. J )
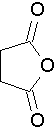
succinic anhydride (Try to name this anhydride by the proper name. J )
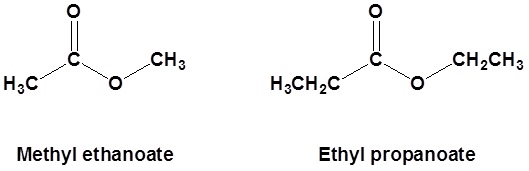
Nomenclature of Esters
Esters are made from a carboxylic acid and an alcohol.
Esters are named as if the alkyl chain from the alcohol is a substituent. No number is assigned to this alkyl chain. This is followed by the name of the parent chain form the carboxylic acid part of the ester with an –e remove and replaced with the ending –oate.
| Example 1: |
|---|
 |
Nomenclature of amides
Primary amides
Primary amides are named by changing the name of the acid by dropping the -oic acid or -ic acid endings and adding -amide. The carbonyl carbon is given the #1 location number. It is not necessary to include the location number in the name because it is assumed that the functional group will be on the end of the parent chain.
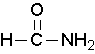
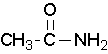
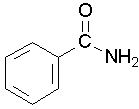
methanamide or formamide (left), ethanamide or acetamide (center) , benzamide (right)
Try to draw a structure for the following compound
- 3-chlorobenzamide J
Try to name the following compound
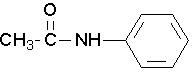
Secondary amides
Secondary amides are named by using an upper case N to designate that the alkyl group is on the nitrogen atom. Alkyl groups attached to the nitrogen are named as substituents. The letter N is used to indicate they are attached to the nitrogen. Tertiary amides are named in the same way.
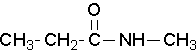
N-methylpropanamide
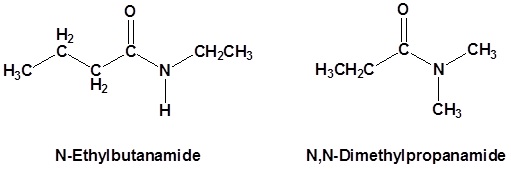
Try to draw a structure for the following compound
- N,N-dimethylformamide J
Try to name the following compound
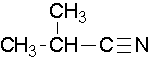
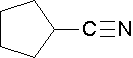
Name the parent alkane (include the carbon atom of the nitrile as part of the parent) followed with the word -nitrile. The carbon in the nitrile is given the #1 location position. It is not necessary to include the location number in the name because it is assumed that the functional group will be on the end of the parent chain.
Nomenclature of nitriles
Cycloalkanes are followed by the word -carbonitrile. The substituent name is cyano.
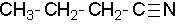
- 1-butanenitrile or 1-cyanopropane
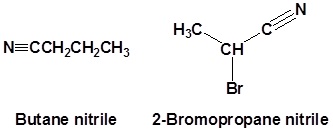
Try to name the following compounds using these conventions�
Try to draw structures for the following compounds�
Some common names that you should know are...
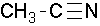 acetonitrile
acetonitrile
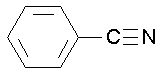 benzonitrile
benzonitrile
Try to draw a structure for the following compound�
- 2-methoxybenzonitrile J