15.8 Radical Halogenation at Allylic Carbon
- Page ID
- 28317
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)When halogens are in the presence of unsaturated molecules such as alkenes, the expected reaction is addition to the double bond carbons resulting in a vicinal dihalide (halogens on adjacent carbons). However, when the halogen concentration is low enough, alkenes containing allylic hydrogens undergo substitution at the allylic position rather than addition at the double bond. The product is an allylic halide (halogen on carbon next to double bond carbons), which is acquired through a radical chain mechanism.
![]()
Why Substitution of Allylic Hydrogens?
As the table below shows, the dissociation energy for the allylic C-H bond is lower than the dissociation energies for the C-H bonds at the vinylic and alkylic positions. This is because the radical formed when the allylic hydrogen is removed is resonance-stabilized. Hence, given that the halogen concentration is low, substitution at the allylic position is favored over competing reactions. However, when the halogen concentration is high, addition at the double bond is favored because a polar reaction outcompetes the radical chain reaction.
Radical Allylic Bromination (Wohl-Ziegler Reaction)
Preparation of Bromine (low concentration)
NBS (N-bromosuccinimide) is the most commonly used reagent to produce low concentrations of bromine. When suspended in tetrachloride (CCl4), NBS reacts with trace amounts of HBr to produce a low enough concentration of bromine to facilitate the allylic bromination reaction.
.jpg?revision=1&size=bestfit&width=307&height=104)
Allylic Bromination Mechanism
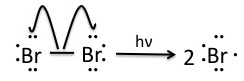
Step 1: Initiation
Once the pre-initiation step involving NBS produces small quantities of Br2, the bromine molecules are homolytically cleaved by light to produce bromine radicals.
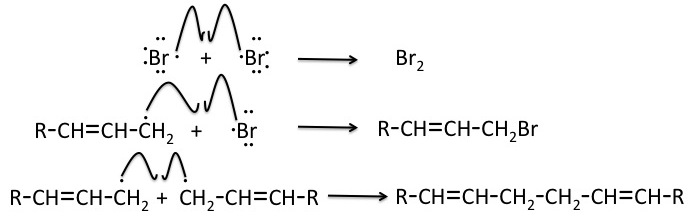
Step 2: Propagation
One bromine radical produced by homolytic cleavage in the initiation step removes an allylic hydrogen of the alkene molecule. A radical intermediate is generated, which is stabilized by resonance. The stability provided by delocalization of the radical in the alkene intermediate is the reason that substitution at the allylic position is favored over competing reactions such as addition at the double bond.
.jpg?revision=1)
The intermediate radical then reacts with a Br2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain mechanism. If the alkene reactant is asymmetric, two distinct product isomers are formed.
.jpg?revision=1)
Step 3: Termination
The radical chain mechanism of allylic bromination can be terminated by any of the possible steps shown below.
Radical Allylic Chlorination
Like bromination, chlorination at the allylic position of an alkene is achieved when low concentrations of Cl2 are present. The reaction is run at high temperatures to achieve the desired results.
Industrial Uses
Allylic chlorination has important practical applications in industry. Since chlorine is inexpensive, allylic chlorinations of alkenes have been used in the industrial production of valuable products. For example, 3-chloropropene, which is necessary for the synthesis of products such as epoxy resin, is acquired through radical allylic chlorination (shown below).
![]()
Problems (Answers are attached as a file)
- Cyclooctene undergoes radical allylic bromination. Write out the complete mechanism including reactants, intermediates and products.
- Predict the two products of the allylic chlorination reaction of 1-heptene.
- What conditions are required for allylic halogenation to occur? Why does this reaction outcompete other possible reactions such as addition when these conditions are met?
- Predict the product of the allylic bromination reaction of 2-benzylheptane. (Hint: How are benzylic hydrogens similar to allylic hydrogens?)
- The reactant 5-isopropyl-1-hexene generates the products 3-bromo-5-isopropyl-1-hexene and 1-bromo-5-isopropyl-2-hexene. What reagents were used in this reaction?
References
- Djerassi, Carl. "Brominations with N-Bromosuccinimide and Related Compounds - The Wohl-Ziegler Reaction." Chemical Reviews 43 (1948):271-314.
- Easton, Christopher J., Alison J. Edwards, Stephen B. McNabb, Martin C. Merrett, Jenny L. O'Connell, Gregory W. Simpson, Jamie S. Simpson, and Anthony C. Willis. "Allylic halogenation of unsaturated amino acids." Organic and Biomolecular Chemistry (2003). RSC Publishing. 9 June 2003. Royal Society of Chemistry. 25 Feb. 2009.
- Kent, Doug. Allylic Bromination. Chem 118B Workshop. Learning Skills Center. 3 Feb. 2009.
- Li, Chao-Jun, and Tak-Hang Chan. Comprehensive Organic Reactions in Aqueous Media. New York: Wiley-Interscience, 2007.
- Vollhardt, Peter C., and Neil E. Schore. Organic Chemistry: Structure and Function. 5th ed. New York: W.H. Freeman and Company, 2007.
Outside Links
- http://en.wikipedia.org/wiki/N-Bromosuccinimide#Preparation
- http://en.wikipedia.org/wiki/Wohl-Ziegler_reaction
- http://www.mhhe.com/physsci/chemistry/carey/student/olc/graphics/carey04oc/ref/ch10allylic.html
Contributors:
- Layne A. Morsch (University of Illinois Springfield)


