3.5: Ionic Nomenclature
- Page ID
- 490079
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To use the rules for naming ionic compounds
After learning a few more details about the names of individual ions, you will be a step away from knowing how to name ionic compounds. This section begins the formal study of nomenclature, the systematic naming of chemical compounds.
Naming Ions
The name of a monatomic cation is simply the name of the element followed by the word ion. Thus, Na+ is the sodium ion, Al3+ is the aluminum ion, Ca2+ is the calcium ion, and so forth.
We have seen that some elements lose different numbers of electrons, producing ions of different charges. Iron, for example, can form two cations, each of which, when combined with the same anion, makes a different compound with unique physical and chemical properties. Thus, we need a different name for each iron ion to distinguish Fe2+ from Fe3+. The same issue arises for other ions with more than one possible charge.
To distinguish these different ions, an ion’s positive charge is indicated by a roman numeral in parentheses after the element name, followed by the word ion. Thus, Fe2+ is called the iron(II) ion, while Fe3+ is called the iron(III) ion. This system is used only for elements that form more than one common positive ion. We do not call the Na+ ion the sodium(I) ion because (I) is unnecessary. Sodium forms only a 1+ ion, so there is no ambiguity about the name sodium ion.
The name of a monatomic anion consists of the stem of the element name, the suffix -ide, and then the word ion. Thus, as we have already seen, Cl− is “chlor-” + “-ide ion,” or the chloride ion. Similarly, O2− is the oxide ion, Se2− is the selenide ion, and so forth. Table \(\PageIndex{2}\) lists the names of some common monatomic ions.
| Ion | Name |
|---|---|
| F− | fluoride ion |
| Cl− | chloride ion |
| Br− | bromide ion |
| I− | iodide ion |
| O2− | oxide ion |
| S2− | sulfide ion |
| P3− | phosphide ion |
| N3− | nitride ion |
The polyatomic ions have their own characteristic names, as discussed earlier.
Name each ion.
- Ca2+
- S2−
- SO32−
- NH4+
- Cu+
- Answer a
-
the calcium ion
- Answer b
-
the sulfide ion (from Table \(\PageIndex{2}\) )
- Answer c
-
the sulfite ion
- Answer d
-
the ammonium ion
- Answer e
-
the copper(I) ion. Copper can form cations with either a 1+ or 2+ charge, so we have to specify which the charge of the ion
Name each ion.
- Fe2+
- Fe3+
- SO42−
- Ba2+
- HCO3−
- Answer a
-
the iron (II)
- Answer b
-
the iron (III)
- Answer c
-
the sulfate ion
- Answer d
-
the barium ion
- Answer e
-
the bicarbonate ion or hydrogen carbonate ion
Write the formula for each ion.
- the bromide ion
- the phosphate ion
- the copper(II) ion
- the magnesium ion
- Answer a
-
Br−
- Answer b
-
PO43−
- Answer c
-
Cu2+
- Answer d
-
Mg2+
Write the formula for each ion.
- the fluoride ion
- the carbonate ion
- the iron(II) ion
- the potassium ion
- Answer a
-
F−
- Answer b
-
CO32-
- Answer c
-
Fe2+
- Answer d
-
K+
Naming Compounds
Now that we know how to name ions, we are ready to name ionic compounds. We do so by placing the name of the cation first, followed by the name of the anion, and dropping the word ion from both parts. For example, what is the name of the compound whose formula is \(\ce{Ba(NO3)2}\)?
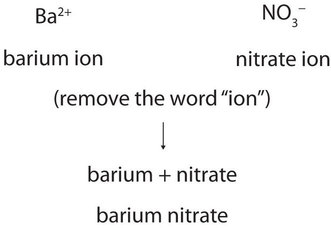
The compound’s name does not indicate that there are two nitrate ions for every barium ion. You must determine the relative numbers of ions by balancing the positive and negative charges.
If you are given a formula for an ionic compound whose cation can have more than one possible charge, you must first determine the charge on the cation before identifying its correct name. For example, consider \(\ce{FeCl2}\) and \(\ce{FeCl3}\). In the first compound, the iron ion has a 2+ charge because there are two \(\ce{Cl^{−}}\) ions in the formula (1− charge on each chloride ion). In the second compound, the iron ion has a 3+ charge, as indicated by the three \(\ce{Cl^{−}}\) ions in the formula.
Name each ionic compound, using Roman numerals if necessary.
- Ca3(PO4)2
- (NH4)2Cr2O7
- KCl
- CuCl
- SnF2
- Answer a
-
calcium phosphate
- Answer b
-
ammonium dichromate (the prefix di- is part of the name of the anion)
- Answer c
-
potassium chloride
- Answer d
-
copper(I) chloride
- Answer e
-
tin(II) fluoride
Name each ionic compound, using Roman numerals if necessary.
- ZnBr2
- Fe(NO3)3
- Al2O3
- CuF2
- AgF
- Answer a
-
zinc bromide
- Answer b
-
iron (III) nitrate
- Answer c
-
aluminum oxide
- Answer d
-
copper (II) fluoride
- Answer e
-
silver fluoride
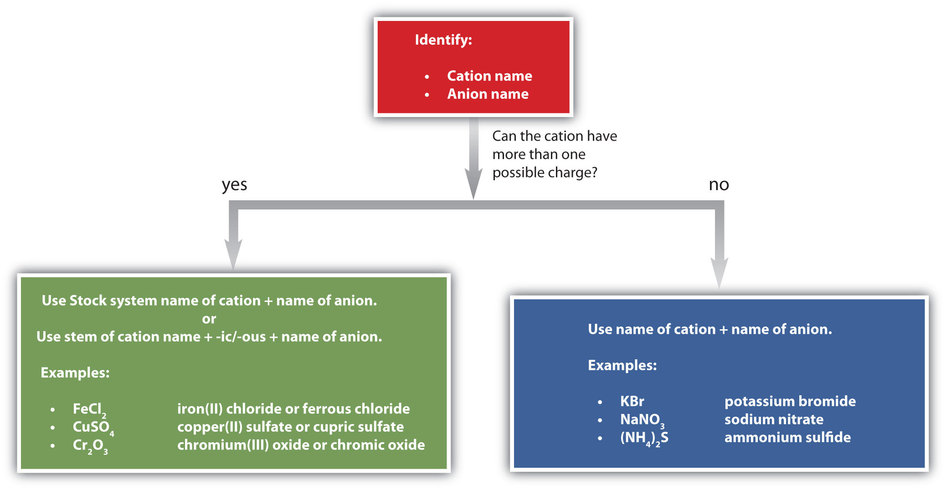
Figure \(\PageIndex{1}\) is a synopsis of how to name simple ionic compounds.
KEY TAKEAWAY
- Each ionic compound has its own unique name that comes from the names of the ions.
EXERCISES
- Briefly describe the process for naming an ionic compound.
- In what order do the names of ions appear in the names of ionic compounds?
- Name each ion.
- Ra2+
- P3−
- H2PO4−
- Sn4+
4. Name each ion.
- Cs+
- As3−
- HSO4−
- Sn2+
5. Name the ionic compound formed by each pair of ions.
- Na+ and Br−
- Mg2+ and Br−
- Mg2+ and S2−
6. Name the ionic compound formed by each pair of ions.
- K+ and Cl−
- Mg2+ and Cl−
- Mg2+ and Se2−
7. Name the ionic compound formed by each pair of ions.
- Na+ and N3−
- Mg2+ and N3−
- Al3+ and S2−
8. Name the ionic compound formed by each pair of ions.
- Li+ and N3−
- Mg2+ and P3−
- Li+ and P3−
9. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Fe3+ and Br−
- Fe2+ and Br−
- Au3+ and S2−
- Au+ and S2−
10. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Cr3+ and O2−
- Cr2+ and O2−
- Pb2+ and Cl−
- Pb4+ and Cl−
11. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Cr3+ and NO3−
- Fe2+ and PO43−
- Ca2+ and CrO42−
- Al3+ and OH−
12. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- NH4+ and NO3−
- K+ and Cr2O72−
- Cu+ and CO32−
- Na+ and HCO3−
13. Give two names for each compound.
- Al(HSO4)3
- Mg(HSO4)2
14. Give two names for each compound.
- Co(HCO3)2
- LiHCO3
Answers
- Name the cation and then the anion but don’t use numerical prefixes.
- the cation name followed by the anion name
3.
- the radium ion
- the phosphide ion
- the dihydrogen phosphate ion
- the tin(IV) ion
4.
- the cesium ion
- the arsenide ion
- the hydrogen sulfate ion
- the tin(II) ion
5.
- sodium bromide
- magnesium bromide
- magnesium sulfide
6.
- potassium chloride
- magnesium chloride
- magnesium selenide
7.
- sodium nitride
- magnesium nitride
- aluminum sulfide
8.
- lithium nitride
- magnesium phosphide
- lithium phosphide
9.
- iron(III) bromide
- iron(II) bromide
- gold(III) sulfide
- gold(I) sulfide
10.
- chromium(III) oxide
- chromium(II) oxide
- lead(II) chloride
- lead(IV) chloride
11.
- chromium(III) nitrate
- iron(II) phosphate
- calcium chromate
- aluminum hydroxide
12.
- ammonium nitrate
- potassium dichromate
- copper(I) carbonate
- sodium hydrogen carbonate or sodium bicarbonate
13.
- aluminum hydrogen sulfate or aluminum bisulfate
- magnesium hydrogen sulfate or magnesium bisulfate
14.
- cobalt hydrogen carbonate or cobalt bicarbonate
- lithium hydrogen carbonate or lithium bicarbonate


