10: Fundamentals of Nuclear Chemistry (WorkSheet)
- Page ID
- 81989
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Using the conservation laws to find an unknowns in a nuclear reaction equation
- Write a balanced nuclear equation for an natural transmutations
Nuclear reactions are going on all around us. Using correctly balanced equations is important whetting to understand nuclear reactions. All equations need to be balance to conform to two conservation laws: The mass number and the electrical charge.
Success Criteria
- Balance nuclear reactions
- Identify the products of nuclear reactions
- Recognize the three primary modes of radioatciity
- Identify stability via the Belt of Stability
Nuclear Reactions
Nuclear chemistry is the subfield of chemistry dealing with radioactivity, nuclear processes, such as nuclear transmutation, and nuclear properties. It is the chemistry of radioactive elements such as the actinides, radium and radon together with the chemistry associated with equipment (such as nuclear reactors) which are designed to perform nuclear processes. This includes the corrosion of surfaces and the behavior under conditions of both normal and abnormal operation (such as during an accident). An important area is the behavior of objects and materials after being placed into a nuclear waste storage or disposal site.
As you remember from Chem 2A, an atom is composed of a nucleus and 1 or more electrons. While, the nucleus has 1/100,000 the radius of the atom, it has nearly all its mass. Nuclei are composed of positively charged protons and neutral neutrons. Elements are distinguished by the atomic number \(Z\) which quantifies the number of protons. Similarly, the \(N\) refers number of neutrons in a nucleus. The mass number (\(A\)) is the total number of protons \(Z\) and neutrons \(N\)
\[A = Z + N\]
An isotope is a nucleus with the same \(Z\) as another nucleus, but with a different neutron number \(N\). Isotopes are nearly identical and have the same number of protons, e.g. U-235, U-236, and U-238 refer to three isotopes of uranium with mass numbers of 235, 236 and 238. A nuclear nomenclature can be adopted to describe an specific nucleus that explicitly indicates both \(A\) and \(Z\):
\[ \ce{^{A}_{Z} Element\, Symbol}\]
Within this nomenclature, the U-235, U-236, and U-238 nuclei would be written as \( \ce{^{235}_{92} U}\), \( \ce{^{236}_{92} U}\), and \( \ce{^{238}_{92} U}\), respectively. Note that \(Z\) does not change for isotopes of the same element.
The nuclear nomenclature can be used to describe more than isotopes. for example, the \(\ce{^4_2He}\) is a helium nucleus and is also known as an alpha particle. Similarly, an electron is written as \(\ce{^0_{-1}e}\) and is known as a beta particle when emitted by a nucleus. In a nuclear decay reaction, also called radioactive decay, an unstable nucleus emits radiation and is transformed into the nucleus of one or more other elements. The resulting daughter nuclei have a lower mass and are lower in energy (more stable) than the parent nucleus that decayed.
Q1
The following are two nuclear decay reactions
\[\ce{^{220}_{87}Fr} \rightarrow \ce{^{4}_{2}He } + \ce{^{216}_{85}At} \label{eq1}\]
\[\ce{^{16}_{7}N} \rightarrow \ce{^{0}_{-1}e} + \ce{^{16}_{8}O} \label{eq2}\]
It is common that the electric charges is not indicated (but can be).
- What are the products of the decay of Francium-220?
- What are the products of the decay of Nitrogen-16?
- Is an alpha particle gained or released in the decay of Francium-220?
- Is an electron gained or released in the decay of Nitrogen-16?
- What is the mass number of an alpha particle?
- What is the charge of an alpha particle?
- What is the mass number of a beta particle?
- What is the charge of an beta particle?
Q2
By examining Equations \(\ref{eq1}\) and \(\ref{eq2}\), what is the mathematical relationship between the total mass number of the reactants and the total mass number of the products. Show work.
Q3
By examining Equations \(\ref{eq1}\) and \(\ref{eq2}\), what is the mathematical relationship between the total charge of the reactants and the total charge of the products. Show work.
Radioactivity
A nucleus that is not permanently stable is radioactive and eventually decays into another. Although the decay of a particular radioactive nucleus is random, 50% of a collection of radioactive nuclei decays in one half-life (\(t_{1/2}\)), just like in chemical chemistry. There are various ways in which nuclei can decay through emitting radiation
Alpha Decay: Alpha decay only occurs in the heaviest nuclei with \(Z > 83\) or \(A ≥ 200\). As an alpha particle is a helium nucleus 4He, the mass number of the daughter nucleus is reduced by 4 and the atomic number (Z) is reduced by 2. Examples of alpha decay are
\[\ce{^{238}_{92}U} → \ce{^{234}_{90}Th} + \alpha\]
with a \(t_{1/2} = 4 \times 10^9\, \text{years}\) or
\[\ce{^{216}_{86}Rn} →\ce{^{212}_{84}Po} + \alpha\]
with \(t_{1/2} = 45 \times 10^{-6}\, \text{sec}\).
Alpha particles are stopped by a few cm of air, a sheet of paper or human skin.
Beta Decay: Beta decay occurs for all values of mass number. It is the emission of an electron (known as \(β^-\) decay) or a positron (known as \(β^+\) decay) with a resulting change in atomic number, \(Z\), but not mass number, \(A\). Neutrons or protons in the nucleus decay by emitting electrons via the weak interaction. This decay is always accompanied by emission of a neutrino or anti-neutrino (a massless particle with no charge).
- \(β^-\) Decay
\[\ce{^{1}_0 n} → \ce{^{1}_1p^+} + \ce{^{0}_{-1} β^{-}} + \text{anti-neutrino}\]
\[\ce{^{27}_{12}Mg} → \ce{^{27}_{13}Al}^{*} + \ce{^{0}_{-1} β^{-}} + \text{anti-neutrino}\]
The \(*\) is meant to signify that the nucleus is unstable (e. g., in the excited-state) and will also decay (e. g., via gamma decay).
- \(β^+\) Decay
\[\ce{^{27}_{14}Si} → \ce{^{27}_{13}Al}^* + \ce{^{0}_{+1} β^{+}} + \text{neutrino}\]
Beta radiation is stopped by a thin piece of wood or plastic.
Gamma Decay: Gamma radiation is high energy electromagnetic particles/waves. They are emitted by nuclei in an excited state attempting to return to their ground state.
\[\ce{^{27}_{13}Al}^{*} \rightarrow \ce{^{27}_{13}Al} + \gamma\]
There is no accompanying change in their mass number, A, or the atomic number, Z, with a half-llfe usually less than \(1\,µs\). Gamma radiation can only be stopped by a substantial thickness of heavy material such as lead.
Q4
Use the laws of conservation of \(A\), \(N\) and \(Z\) and charge to determine the identify of \(X\) in the nuclear reaction equations below (include both \(Z\) and \(A\) for \(X\). You will need to refer to a periodic table.
- \(\ce{^{222}_{86} Rn} \rightarrow \ce{^{4}_{2} He} + X\)
- \(\ce{^{14}_{6}C} \rightarrow \ce{^{0}_{-1} e^{-}} + X\)
- \(\ce{X} \rightarrow \ce{^{0}_{+1} e^{+}} + \ce{^{19}_{9} F} \)
Q5
Write the balanced equation for the beta decay of Sr-90.
Belt of Stability
The graph of stable elements is commonly referred to as the Band (or Belt) of Stability. The graph consists of a y-axis labeled neutrons, an x-axis labeled protons, and a nuclei. At the higher end (upper right) of the band of stability lies the radionuclides that decay via alpha decay, below is positron emission or electron capture, above is beta emissions and elements beyond the atomic number of 83 are only unstable radioactive elements. Stable nuclei with atomic numbers up to about 20 have an neutron:proton ratio of about 1:1 (solid line).
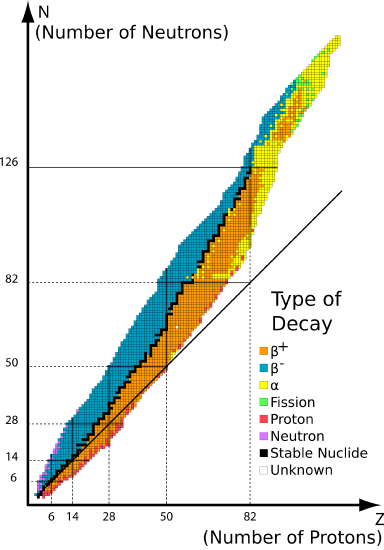
Q6
Find the reactions from Q4 and Q5 on the graph above. Where are they located on the belt (above, below or on the belt)?
Q7
The nuclei on the belt are stable and those off the belt are unstable. Hence to move a nucleus from on the belt to off requires energy (i.e., not spontaneous) and vice versa. Are the decays in Q4 and Q5 spontaneous or non-spontaneous?
Additional Questions
Q8
Identify the following as Alpha, beta, gamma or neutron:
- \(\ce{^1_0 n}\)
- \(\ce{^0_{-1} e}\)
- \(\ce{^4_2 He^{2+}}\)
- \(\ce{^0_0 \gamma}\)
- Nuclear decay with no mass nor charge:
- An electron
- Least penetrating nuclear decay
- Most damaging nuclear decay to the human body
- Nuclear decay that can be stopped by skin paper
- Nuclear decay that can be stopped by aluminum
Q9
How many protons, neutrons, and electrons are in \(\ce{^{195}Pt^{+2}}\)?
Q10
\(\ce{^{62}Cr}\) decays via beta emission. Which statement is correct concerning \(\ce{^{62}Cr}\)?
- The number of electrons decrease.
- The number of protons decrease.
- The number of protons increase.
- The number of neutrons decrease.
- The number of protons increase and the number of neutrons decrease.
Q11
Complete the following nuclear equations (the question marks)
- \(\ce{^{42}_{19}K \rightarrow ^{0}_{-1}e^- + ?}\)
- \(\ce{^{239}_{94}Pu \rightarrow ^{4}_{2}He^{2+} + ?}\)
- \(\ce{^{9}_{4}Be \rightarrow ^{9}_{4}Be} \,+ \, ?\)
- \(\ce{^{235}_{92}U \rightarrow ? + ^{231}_{90}Th}\)
- \(\ce{^{6}_{3}Li \rightarrow ^{4}_{2}He^{2+}}\, + \, ?\)
- \(\ce{? \rightarrow ^{142}_{56}Ba + ^{91}_{36}Kr + 3\; ^{1}_{0}n}\)
Q12
Write equations for the following nuclear decay reactions. Make sure that both mass numbers and atomic numbers are balanced on each side
- Decay of polonium-218 by alpha emission.
- Decay of carbon-14 by beta \(\beta^-\) emission.
- The alpha decay of radon-198
- The beta \(\beta^-\) decay of uranium-237
Decay Chain
In nuclear science, the decay chain refers to the radioactive decay of different discrete radioactive decay products as a chained series of transformations. They are also known as "radioactive cascades". Most radioisotopes do not decay directly to a stable state, but rather undergo a series of decays until eventually a stable isotope is reached (i.e., a nucleus on the belt of stability). Decay stages are referred to by their relationship to previous or subsequent stages. A parent isotope is one that undergoes decay to form a daughter isotope. One example of this is uranium (atomic number 92) decaying into thorium (atomic number 90). The daughter isotope may be stable or it may decay to form a daughter isotope of its own. The daughter of a daughter isotope is sometimes called a granddaughter isotope.
Q13
The figure below maps the radioactive decay of \(\ce{^{238}U}\) into \(\ce{^{206}Pb}\). Use this figure to answer the following three questions:
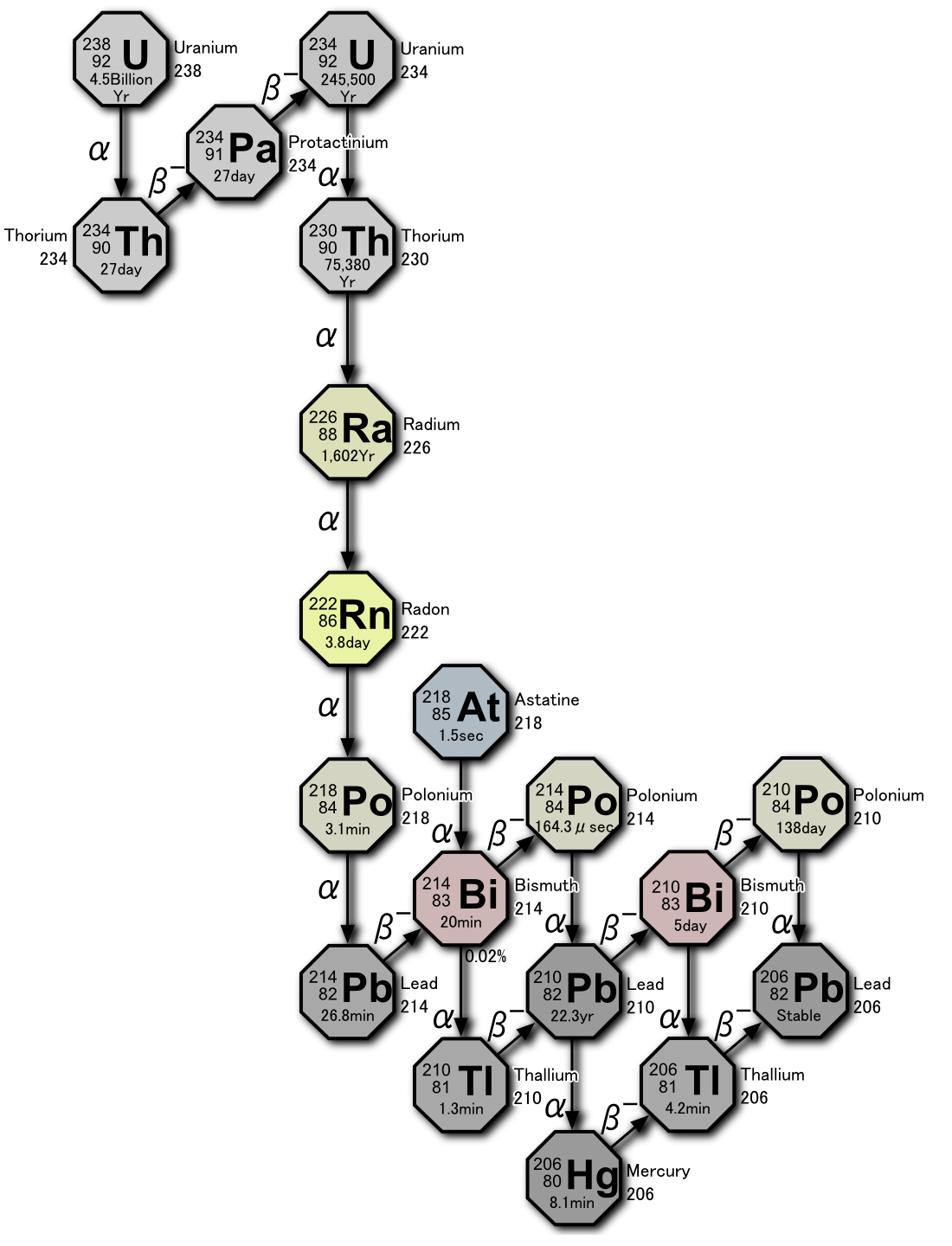
- How many alpha particles are produced as one atom of \(\ce{^{238}U}\) decays to one atom of \(\ce{^{206}Pb}\)? Draw the decay pathway you used for this calculation? Does it change if you picked a different pathway?
- How many beta particles are produced as one atom of \(\ce{^{238}U}\) decays to one atom of \(\ce{^{206}Pb}\)? Draw the decay pathway you used for this calculation? Does it change if you picked a different pathway?
- What is the final product in the decay series of \(\ce{^{238}U}\)?
Q14
- Write the nuclear equation showing that when \(\ce{^{229}Pa}\) goes through two consecutive alpha decays to form \(\ce{^{221}Fr}\).
- Write the nuclear equation showing that when \(\ce{^{210}Po}\) goes through two consecutive alpha decays and then a beta decay and then another alpha decay.
Q15
Throium-232 undergoes radioactive decay until a stable isotope is reached. Write the nuclear reaction for each of the 11 steps in the decay of \(\ce{^{238}Th}\) with each product becoming the reactant of the next decay. What is the final stable isotope?
- Step 1: Alpha decay
- Step 2: Beta decay
- Step 3: Beta decay
- Step 4: Alpha decay
- Step 5: Alpha decay
- Step 6: Alpha decay
- Step 7: Alpha decay
- Step 8: Beta decay
- Step 9: Beta decay
- Step 10: Alpha decay
- Step 11: Beta decay


