3.1a: Chemical Compounds
- Page ID
- 36978
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To understand the differences between covalent and ionic bonding.
The atoms in all substances that contain multiple atoms are held together by electrostatic interactions—interactions between electrically charged particles such as protons and electrons. Electrostatic attraction between oppositely charged species (positive and negative) results in a force that causes them to move toward each other, like the attraction between opposite poles of two magnets. In contrast, electrostatic repulsion between two species with the same charge (either both positive or both negative) results in a force that causes them to repel each other, as do the same poles of two magnets. Atoms form chemical compounds when the attractive electrostatic interactions between them are stronger than the repulsive interactions. Collectively, the attractive interactions between atoms are called chemical bonds.
Chemical bonds are generally divided into two fundamentally different types: ionic and covalent. In reality, however, the bonds in most substances are neither purely ionic nor purely covalent, but lie on a spectrum between these extremes. Although purely ionic and purely covalent bonds represent extreme cases that are seldom encountered in any but very simple substances, a brief discussion of these two extremes helps explain why substances with different kinds of chemical bonds have very different properties. Ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist of molecules, which are groups of atoms in which one or more pairs of electrons are shared between bonded atoms. In a covalent bond, atoms are held together by the electrostatic attraction between the positively charged nuclei of the bonded atoms and the negatively charged electrons they share. This discussion of structures and formulas begins by describing covalent compounds. The energetic factors involved in bond formation are described in more quantitative detail in later.
Ionic compounds consist of ions of opposite charges held together by strong electrostatic forces, whereas pairs of electrons are shared between bonded atoms in covalent compounds.
Covalent Molecules and Compounds
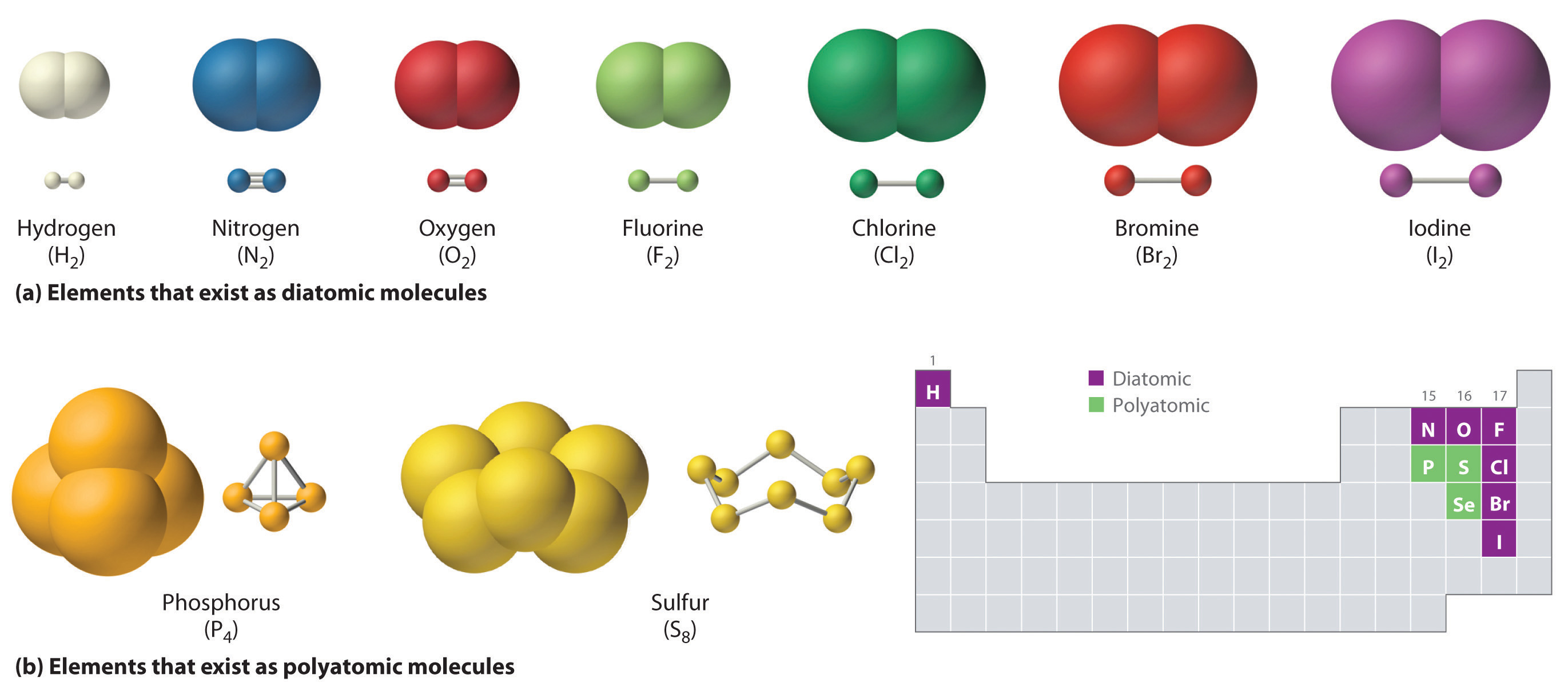
Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the fundamental chemical properties of a covalent compound. Some pure elements exist as covalent molecules. Hydrogen, nitrogen, oxygen, and the halogens occur naturally as the diatomic (“two atoms”) molecules H2, N2, O2, F2, Cl2, Br2, and I2 (part (a) in Figure \(\PageIndex{1}\)). Similarly, a few pure elements exist as polyatomic (“many atoms”) molecules, such as elemental phosphorus and sulfur, which occur as P4 and S8 (part (b) in Figure \(\PageIndex{1}\)).
Each covalent compound is represented by a molecular formula, which gives the atomic symbol for each component element, in a prescribed order, accompanied by a subscript indicating the number of atoms of that element in the molecule. The subscript is written only if the number of atoms is greater than 1. For example, water, with two hydrogen atoms and one oxygen atom per molecule, is written as \(H_2O\). Similarly, carbon dioxide, which contains one carbon atom and two oxygen atoms in each molecule, is written as \(CO_2\).
Covalent compounds that predominantly contain carbon and hydrogen are called organic compounds. The convention for representing the formulas of organic compounds is to write carbon first, followed by hydrogen and then any other elements in alphabetical order (e.g., CH4O is methyl alcohol, a fuel). Compounds that consist primarily of elements other than carbon and hydrogen are called inorganic compounds; they include both covalent and ionic compounds. In inorganic compounds, the component elements are listed beginning with the one farthest to the left in the periodic table, as in CO2 or SF6. Those in the same group are listed beginning with the lower element and working up, as in ClF. By convention, however, when an inorganic compound contains both hydrogen and an element from groups 13–15, hydrogen is usually listed last in the formula. Examples are ammonia (NH3) and silane (SiH4). Compounds such as water, whose compositions were established long before this convention was adopted, are always written with hydrogen first: Water is always written as H2O, not OH2. The conventions for inorganic acids, such as hydrochloric acid (HCl) and sulfuric acid (H2SO4), are described elswhere.
For organic compounds: write C first, then H, and then the other elements in alphabetical order. For molecular inorganic compounds: start with the element at far left in the periodic table; list elements in same group beginning with the lower element and working up.
Write the molecular formula of each compound.
- The phosphorus-sulfur compound that is responsible for the ignition of so-called strike anywhere matches has 4 phosphorus atoms and 3 sulfur atoms per molecule.
- Ethyl alcohol, the alcohol of alcoholic beverages, has 1 oxygen atom, 2 carbon atoms, and 6 hydrogen atoms per molecule.
- Freon-11, once widely used in automobile air conditioners and implicated in damage to the ozone layer, has 1 carbon atom, 3 chlorine atoms, and 1 fluorine atom per molecule.
Given: identity of elements present and number of atoms of each
Asked for: molecular formula
Strategy:
A Identify the symbol for each element in the molecule. Then identify the substance as either an organic compound or an inorganic compound.
B If the substance is an organic compound, arrange the elements in order beginning with carbon and hydrogen and then list the other elements alphabetically. If it is an inorganic compound, list the elements beginning with the one farthest left in the periodic table. List elements in the same group starting with the lower element and working up.
C From the information given, add a subscript for each kind of atom to write the molecular formula.
Solution:
a.
- A The molecule has 4 phosphorus atoms and 3 sulfur atoms. Because the compound does not contain mostly carbon and hydrogen, it is inorganic.
- B Phosphorus is in group 15, and sulfur is in group 16. Because phosphorus is to the left of sulfur, it is written first.
- C Writing the number of each kind of atom as a right-hand subscript gives P4S3 as the molecular formula.
b.
- A Ethyl alcohol contains predominantly carbon and hydrogen, so it is an organic compound.
- B The formula for an organic compound is written with the number of carbon atoms first, the number of hydrogen atoms next, and the other atoms in alphabetical order: CHO.
- C Adding subscripts gives the molecular formula \(\ce{C2H6O}\).
c.
- A Freon-11 contains carbon, chlorine, and fluorine. It can be viewed as either an inorganic compound or an organic compound (in which fluorine has replaced hydrogen). The formula for Freon-11 can therefore be written using either of the two conventions.
- B According to the convention for inorganic compounds, carbon is written first because it is farther left in the periodic table. Fluorine and chlorine are in the same group, so they are listed beginning with the lower element and working up: CClF. Adding subscripts gives the molecular formula CCl3F.
- C We obtain the same formula for Freon-11 using the convention for organic compounds. The number of carbon atoms is written first, followed by the number of hydrogen atoms (zero) and then the other elements in alphabetical order, also giving CCl3F.
Write the molecular formula for each compound.
- Nitrous oxide, also called “laughing gas,” has 2 nitrogen atoms and 1 oxygen atom per molecule. Nitrous oxide is used as a mild anesthetic for minor surgery and as the propellant in cans of whipped cream.
- Sucrose, also known as cane sugar, has 12 carbon atoms, 11 oxygen atoms, and 22 hydrogen atoms.
- Sulfur hexafluoride, a gas used to pressurize “unpressurized” tennis balls and as a coolant in nuclear reactors, has 6 fluorine atoms and 1 sulfur atom per molecule.
Answer:
- N2O
- C12H22O11
- SF6
Representations of Molecular Structures
Molecular formulas give only the elemental composition of molecules. In contrast, structural formulas show which atoms are bonded to one another and, in some cases, the approximate arrangement of the atoms in space. Knowing the structural formula of a compound enables chemists to create a three-dimensional model, which provides information about how that compound will behave physically and chemically.
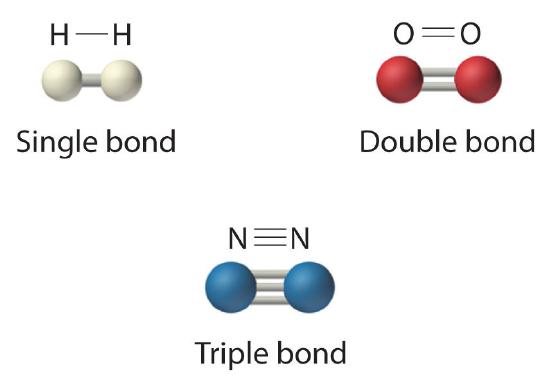
The structural formula for H2 can be drawn as H–H and that for I2 as I–I, where the line indicates a single pair of shared electrons, a single bond. Two pairs of electrons are shared in a double bond, which is indicated by two lines—for example, O2 is O=O. Three electron pairs are shared in a triple bond, which is indicated by three lines—for example, N2 is N≡N (Figure \(\PageIndex{2}\)). Carbon is unique in the extent to which it forms single, double, and triple bonds to itself and other elements. The number of bonds formed by an atom in its covalent compounds is not arbitrary. Hydrogen, oxygen, nitrogen, and carbon have very strong tendencies to form substances in which they have one, two, three, and four bonds to other atoms, respectively (Table \(\PageIndex{1}\)).
| Atom | Number of Bonds |
|---|---|
| H (group 1) | 1 |
| O (group 16) | 2 |
| N (group 15) | 3 |
| C (group 14) | 4 |
The structural formula for water can be drawn as follows:

Because the latter approximates the experimentally determined shape of the water molecule, it is more informative. Similarly, ammonia (NH3) and methane (CH4) are often written as planar molecules:
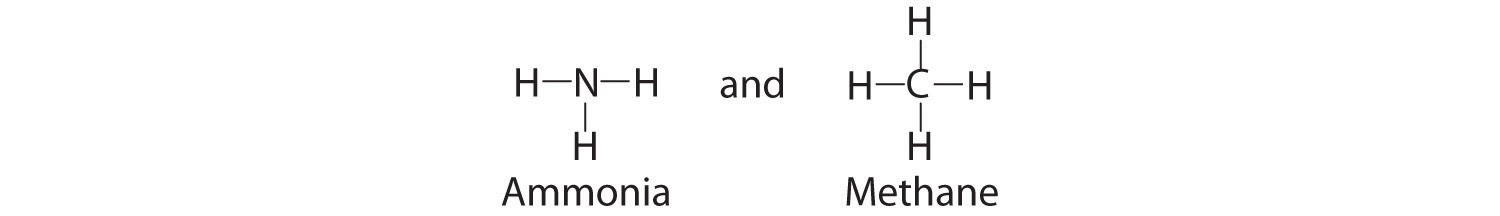
As shown in Figure \(\PageIndex{3}\), however, the actual three-dimensional structure of NH3 looks like a pyramid with a triangular base of three hydrogen atoms. The structure of CH4, with four hydrogen atoms arranged around a central carbon atom as shown in Figure \(\PageIndex{3}\), is tetrahedral: the hydrogen atoms are positioned at every other vertex of a cube. Many compounds—carbon compounds, in particular—have four bonded atoms arranged around a central atom to form a tetrahedron.
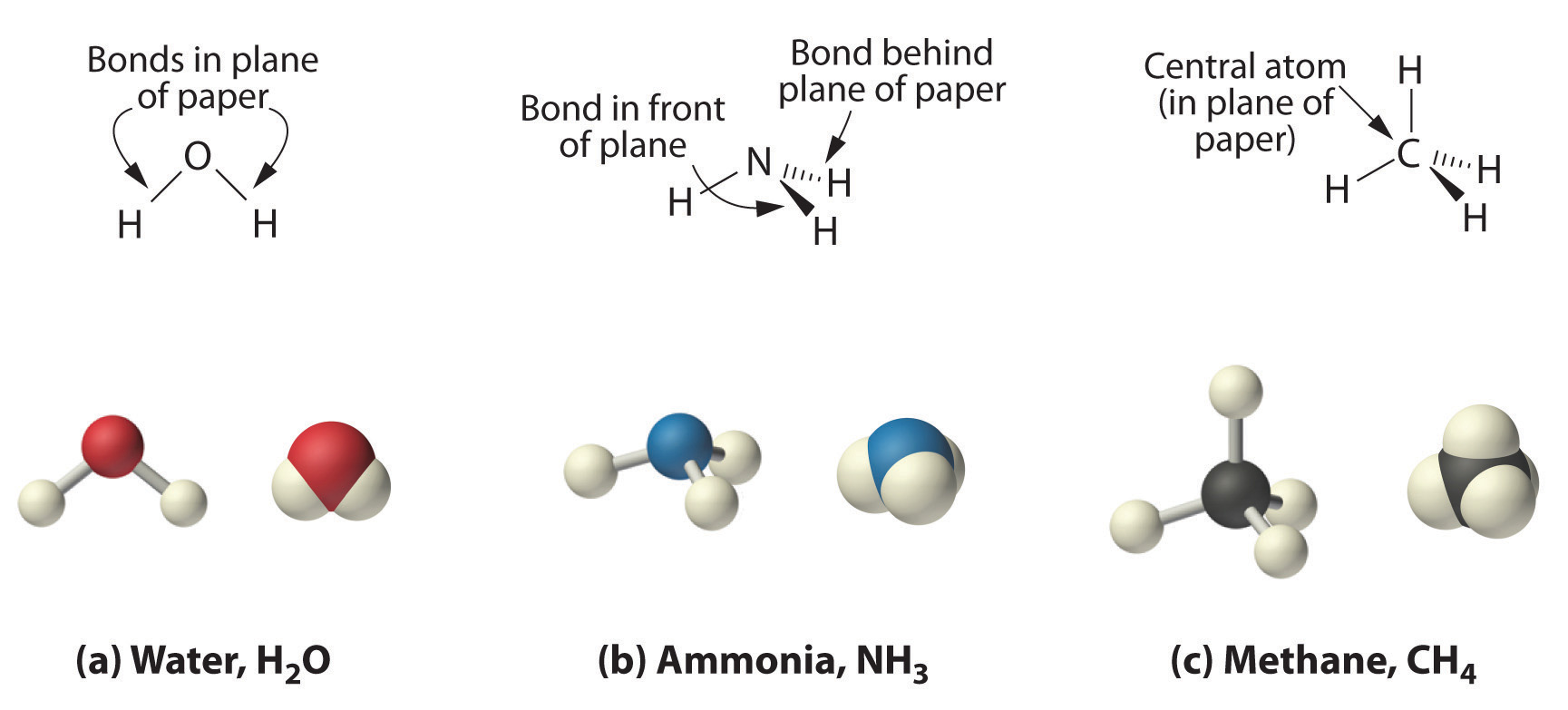
Figures \(\PageIndex{3}\)-\(\PageIndex{3}\) illustrate different ways to represent the structures of molecules. It should be clear that there is no single “best” way to draw the structure of a molecule; the method used depends on which aspect of the structure should be emphasized and how much time and effort is required. Figure \(\PageIndex{4}\) shows some of the different ways to portray the structure of a slightly more complex molecule: methanol. These representations differ greatly in their information content. For example, the molecular formula for methanol (part (a) in Figure \(\PageIndex{4}\)) gives only the number of each kind of atom; writing methanol as CH4O tells nothing about its structure. In contrast, the structural formula (part (b) in Figure \(\PageIndex{4}\)) indicates how the atoms are connected, but it makes methanol look as if it is planar (which it is not). Both the ball-and-stick model (part (c) in Figure \(\PageIndex{4}\)) and the perspective drawing (part (d) in Figure \(\PageIndex{4}\)) show the three-dimensional structure of the molecule. The latter (also called a wedge-and-dash representation) is the easiest way to sketch the structure of a molecule in three dimensions. It shows which atoms are above and below the plane of the paper by using wedges and dashes, respectively; the central atom is always assumed to be in the plane of the paper. The space-filling model (part (e) in Figure \(\PageIndex{4}\)) illustrates the approximate relative sizes of the atoms in the molecule, but it does not show the bonds between the atoms. In addition, in a space-filling model, atoms at the “front” of the molecule may obscure atoms at the “back.”
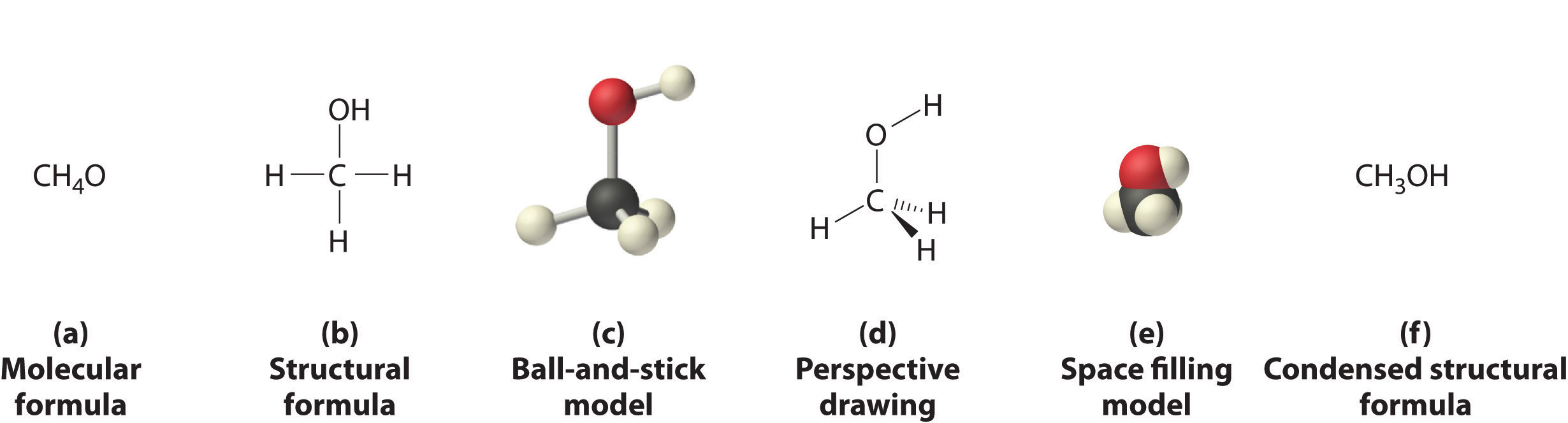
Although a structural formula, a ball-and-stick model, a perspective drawing, and a space-filling model provide a significant amount of information about the structure of a molecule, each requires time and effort. Consequently, chemists often use a condensed structural formula (part (f) in Figure \(\PageIndex{4}\)), which omits the lines representing bonds between atoms and simply lists the atoms bonded to a given atom next to it. Multiple groups attached to the same atom are shown in parentheses, followed by a subscript that indicates the number of such groups. For example, the condensed structural formula for methanol is CH3OH, which indicates that the molecule contains a CH3 unit that looks like a fragment of methane (CH4). Methanol can therefore be viewed either as a methane molecule in which one hydrogen atom has been replaced by an –OH group or as a water molecule in which one hydrogen atom has been replaced by a –CH3 fragment. Because of their ease of use and information content, we use condensed structural formulas for molecules throughout this text. Ball-and-stick models are used when needed to illustrate the three-dimensional structure of molecules, and space-filling models are used only when it is necessary to visualize the relative sizes of atoms or molecules to understand an important point.
Write the molecular formula for each compound. The condensed structural formula is given.
- Sulfur monochloride (also called disulfur dichloride) is a vile-smelling, corrosive yellow liquid used in the production of synthetic rubber. Its condensed structural formula is ClSSCl.
- Ethylene glycol is the major ingredient in antifreeze. Its condensed structural formula is HOCH2CH2OH.
- Trimethylamine is one of the substances responsible for the smell of spoiled fish. Its condensed structural formula is (CH3)3N.
Given: condensed structural formula
Asked for: molecular formula
Strategy:
- Identify every element in the condensed structural formula and then determine whether the compound is organic or inorganic.
- As appropriate, use either organic or inorganic convention to list the elements. Then add appropriate subscripts to indicate the number of atoms of each element present in the molecular formula.
Solution:
The molecular formula lists the elements in the molecule and the number of atoms of each.
- A Each molecule of sulfur monochloride has two sulfur atoms and two chlorine atoms. Because it does not contain mostly carbon and hydrogen, it is an inorganic compound. B Sulfur lies to the left of chlorine in the periodic table, so it is written first in the formula. Adding subscripts gives the molecular formula S2Cl2.
- A Counting the atoms in ethylene glycol, we get six hydrogen atoms, two carbon atoms, and two oxygen atoms per molecule. The compound consists mostly of carbon and hydrogen atoms, so it is organic. B As with all organic compounds, C and H are written first in the molecular formula. Adding appropriate subscripts gives the molecular formula C2H6O2.
- A The condensed structural formula shows that trimethylamine contains three CH3 units, so we have one nitrogen atom, three carbon atoms, and nine hydrogen atoms per molecule. Because trimethylamine contains mostly carbon and hydrogen, it is an organic compound. B According to the convention for organic compounds, C and H are written first, giving the molecular formula C3H9N.

Write the molecular formula for each molecule.
- Chloroform, which was one of the first anesthetics and was used in many cough syrups until recently, contains one carbon atom, one hydrogen atom, and three chlorine atoms. Its condensed structural formula is CHCl3.
- Hydrazine is used as a propellant in the attitude jets of the space shuttle. Its condensed structural formula is H2NNH2.
- Putrescine is a pungent-smelling compound first isolated from extracts of rotting meat. Its condensed structural formula is H2NCH2CH2CH2CH2NH2. This is often written as H2N(CH2)4NH2 to indicate that there are four CH2 fragments linked together.
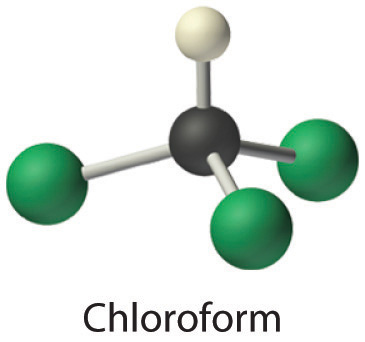
- Answer a
-
CHCl3
- Answer b
-
N2H4
- Answer c
-
C4H12N2
Ionic Compounds
The substances described in the preceding discussion are composed of molecules that are electrically neutral; that is, the number of positively-charged protons in the nucleus is equal to the number of negatively-charged electrons. In contrast, ions are atoms or assemblies of atoms that have a net electrical charge. Ions that contain fewer electrons than protons have a net positive charge and are called cations. Conversely, ions that contain more electrons than protons have a net negative charge and are called anions. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge.
Ionic compounds contain both cations and anions in a ratio that results in zero electrical charge.
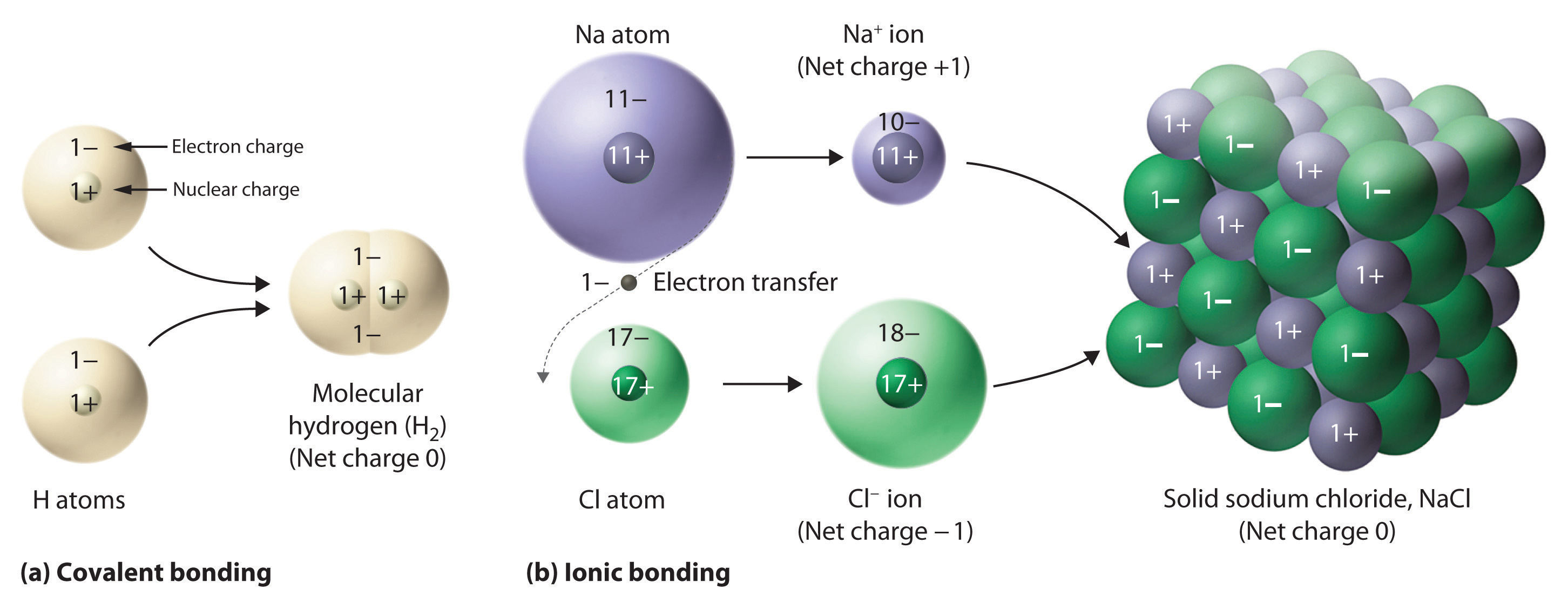
In covalent compounds, electrons are shared between bonded atoms and are simultaneously attracted to more than one nucleus. In contrast, ionic compounds contain cations and anions rather than discrete neutral molecules. Ionic compounds are held together by the attractive electrostatic interactions between cations and anions. In an ionic compound, the cations and anions are arranged in space to form an extended three-dimensional array that maximizes the number of attractive electrostatic interactions and minimizes the number of repulsive electrostatic interactions (Figure \(\PageIndex{5}\)). As shown in Equation 3.1.1, the electrostatic energy of the interaction between two charged particles is proportional to the product of the charges on the particles and inversely proportional to the distance between them:
\[ \text {electrostatic energy} \propto {Q_1Q_2 \over r} \label{3.1.1}\]
where
- \(Q_1\) and \(Q_2\) are the electrical charges on particles 1 and 2, and
- \(r\) is the distance between them.
When \(Q_1\) and \(Q_2\) are both positive, corresponding to the charges on cations, the cations repel each other and the electrostatic energy is positive. When \(Q_1\) and \(Q_2\) are both negative, corresponding to the charges on anions, the anions repel each other and the electrostatic energy is again positive. The electrostatic energy is negative only when the charges have opposite signs; that is, positively charged species are attracted to negatively charged species and vice versa. As shown in Figure \(\PageIndex{6}\), the strength of the interaction is proportional to the magnitude of the charges and decreases as the distance between the particles increases. These energetic factors are discussed in greater quantitative detail later.

If the electrostatic energy is positive, the particles repel each other; if the electrostatic energy is negative, the particles are attracted to each other.
One example of an ionic compound is sodium chloride (NaCl), formed from sodium and chlorine. In forming chemical compounds, many elements have a tendency to gain or lose enough electrons to attain the same number of electrons as the noble gas closest to them in the periodic table. When sodium and chlorine come into contact, each sodium atom gives up an electron to become a Na+ ion, with 11 protons in its nucleus but only 10 electrons (like neon), and each chlorine atom gains an electron to become a Cl− ion, with 17 protons in its nucleus and 18 electrons (like argon), as shown in part (b) in Figure \(\PageIndex{5}\). Solid sodium chloride contains equal numbers of cations (Na+) and anions (Cl−), thus maintaining electrical neutrality. Each Na+ ion is surrounded by 6 Cl− ions, and each Cl− ion is surrounded by 6 Na+ ions. Because of the large number of attractive Na+Cl− interactions, the total attractive electrostatic energy in NaCl is great.
Consistent with a tendency to have the same number of electrons as the nearest noble gas, when forming ions, elements in groups 1, 2, and 3 tend to lose one, two, and three electrons, respectively, to form cations, such as Na+ and Mg2+. They then have the same number of electrons as the nearest noble gas: neon. Similarly, K+, Ca2+, and Sc3+ have 18 electrons each, like the nearest noble gas: argon. In addition, the elements in group 13 lose three electrons to form cations, such as Al3+, again attaining the same number of electrons as the noble gas closest to them in the periodic table. Because the lanthanides and actinides formally belong to group 3, the most common ion formed by these elements is M3+, where M represents the metal. Conversely, elements in groups 17, 16, and 15 often react to gain one, two, and three electrons, respectively, to form ions such as Cl−, S2−, and P3−. Ions such as these, which contain only a single atom, are called monatomic ions. The charges of most monatomic ions derived from the main group elements can be predicted by simply looking at the periodic table and counting how many columns an element lies from the extreme left or right. For example, barium (in Group 2) forms Ba2+ to have the same number of electrons as its nearest noble gas, xenon; oxygen (in Group 16) forms O2− to have the same number of electrons as neon; and cesium (in Group 1) forms Cs+, which has the same number of electrons as xenon. Note that this method is ineffective for most of the transition metals, as discussed in Section 2.3. Some common monatomic ions are listed in Table \(\PageIndex{2}\).
Elements in Groups 1, 2, and 3 tend to form 1+, 2+, and 3+ ions, respectively; elements in Groups 15, 16, and 17 tend to form 3−, 2−, and 1− ions, respectively.
| Group 1 | Group 2 | Group 3 | Group 13 | Group 15 | Group 16 | Group 17 |
|---|---|---|---|---|---|---|
|
Li+ lithium |
Be2+ beryllium |
N3− nitride (azide) |
O2− oxide |
F− fluoride |
||
|
Na+ sodium |
Mg2+ magnesium |
Al3+ aluminum |
P3− phosphide |
S2− sulfide |
Cl− chloride |
|
|
K+ potassium |
Ca2+ calcium |
Sc3+ scandium |
Ga3+ gallium |
As3− arsenide |
Se2− selenide |
Br− bromide |
|
Rb+ rubidium |
Sr2+ strontium |
Y3+ yttrium |
In3+ indium |
Te2− telluride |
I− iodide |
|
|
Cs+ cesium |
Ba2+ barium |
La3+ lanthanum |
Predict the charge on the most common monatomic ion formed by each element.
- aluminum, used in the quantum logic clock, the world’s most precise clock
- selenium, used to make ruby-colored glass
- yttrium, used to make high-performance spark plugs
Given: element
Asked for: ionic charge
Strategy:
A Identify the group in the periodic table to which the element belongs. Based on its location in the periodic table, decide whether the element is a metal, which tends to lose electrons; a nonmetal, which tends to gain electrons; or a semimetal, which can do either.
B After locating the noble gas that is closest to the element, determine the number of electrons the element must gain or lose to have the same number of electrons as the nearest noble gas.
Solution:
- A Aluminum is a metal in group 13; consequently, it will tend to lose electrons. B The nearest noble gas to aluminum is neon. Aluminum will lose three electrons to form the Al3+ ion, which has the same number of electrons as neon.
- A Selenium is a nonmetal in group 16, so it will tend to gain electrons. B The nearest noble gas is krypton, so we predict that selenium will gain two electrons to form the Se2− ion, which has the same number of electrons as krypton.
- A Yttrium is in group 3, and elements in this group are metals that tend to lose electrons. B The nearest noble gas to yttrium is krypton, so yttrium is predicted to lose three electrons to form Y3+, which has the same number of electrons as krypton.
Predict the charge on the most common monatomic ion formed by each element.
- calcium, used to prevent osteoporosis
- iodine, required for the synthesis of thyroid hormones
- zirconium, widely used in nuclear reactors
Answer:
- Ca2+
- I−
- Zr4+
Molecular and Ionic Compounds: https://youtu.be/zJejgCll1bw
Physical Properties of Ionic and Covalent Compounds
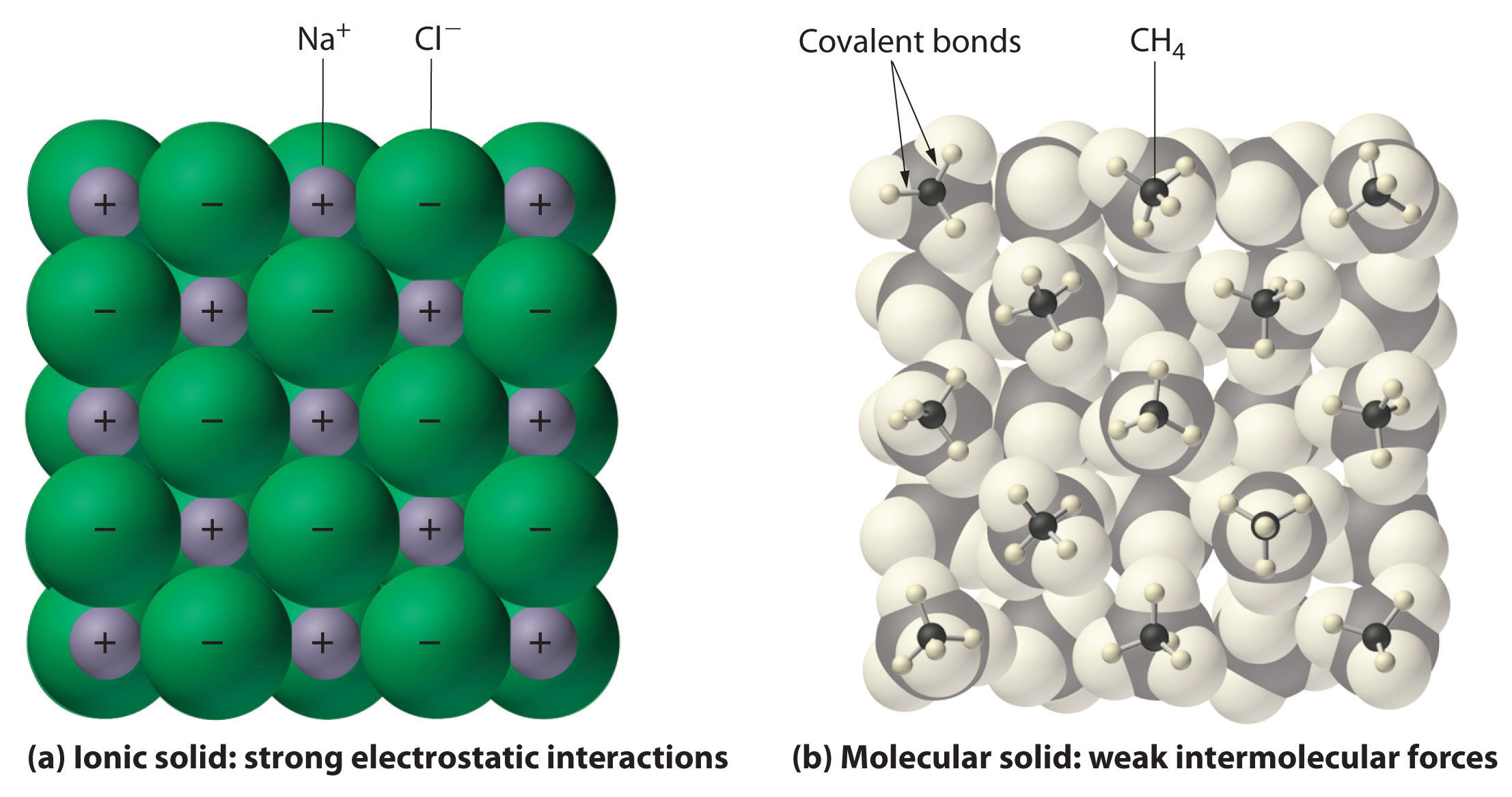
In general, ionic and covalent compounds have different physical properties. Ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. These properties stem from the characteristic internal structure of an ionic solid, illustrated schematically in part (a) in Figure \(\PageIndex{8}\) which shows the three-dimensional array of alternating positive and negative ions held together by strong electrostatic attractions. In contrast, as shown in part (b) in Figure \(\PageIndex{8}\) most covalent compounds consist of discrete molecules held together by comparatively weak intermolecular forces (the forces between molecules), even though the atoms within each molecule are held together by strong intramolecular covalent bonds (the forces within the molecule). Covalent substances can be gases, liquids, or solids at room temperature and pressure, depending on the strength of the intermolecular interactions. Covalent molecular solids tend to form soft crystals that melt at low temperatures and evaporate easily.Some covalent substances, however, are not molecular but consist of infinite three-dimensional arrays of covalently bonded atoms and include some of the hardest materials known, such as diamond. This topic will be addressed elsewhere. The covalent bonds that hold the atoms together in the molecules are unaffected when covalent substances melt or evaporate, so a liquid or vapor of independent molecules is formed. For example, at room temperature, methane, the major constituent of natural gas, is a gas that is composed of discrete CH4 molecules. A comparison of the different physical properties of ionic compounds and covalent molecular substances is given in Table \(\PageIndex{3}\).
| Ionic Compounds | Covalent Molecular Substances |
|---|---|
| hard solids | gases, liquids, or soft solids |
| high melting points | low melting points |
| nonvolatile | volatile |
-
The Learning Objective of this Module is to describe the composition of a chemical compound.
When chemists synthesize a new compound, they may not yet know its molecular or structural formula. In such cases, they usually begin by determining its empirical formula, the relative numbers of atoms of the elements in a compound, reduced to the smallest whole numbers. Because the empirical formula is based on experimental measurements of the numbers of atoms in a sample of the compound, it shows only the ratios of the numbers of the elements present. The difference between empirical and molecular formulas can be illustrated with butane, a covalent compound used as the fuel in disposable lighters. The molecular formula for butane is C4H10. The ratio of carbon atoms to hydrogen atoms in butane is 4:10, which can be reduced to 2:5. The empirical formula for butane is therefore C2H5. The formula unit is the absolute grouping of atoms or ions represented by the empirical formula of a compound, either ionic or covalent. Butane has the empirical formula C2H5, but it contains two C2H5 formula units, giving a molecular formula of C4H10.
Because ionic compounds do not contain discrete molecules, empirical formulas are used to indicate their compositions. All compounds, whether ionic or covalent, must be electrically neutral. Consequently, the positive and negative charges in a formula unit must exactly cancel each other. If the cation and the anion have charges of equal magnitude, such as Na+ and Cl−, then the compound must have a 1:1 ratio of cations to anions, and the empirical formula must be NaCl. If the charges are not the same magnitude, then a cation:anion ratio other than 1:1 is needed to produce a neutral compound. In the case of Mg2+ and Cl−, for example, two Cl− ions are needed to balance the two positive charges on each Mg2+ ion, giving an empirical formula of MgCl2. Similarly, the formula for the ionic compound that contains Na+ and O2− ions is Na2O.
Ionic compounds do not contain discrete molecules, so empirical formulas are used to indicate their compositions.
Binary Ionic Compounds
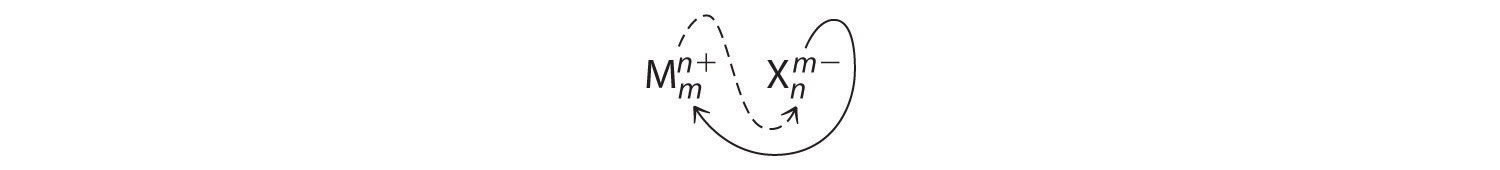
An ionic compound that contains only two elements, one present as a cation and one as an anion, is called a binary ionic compound. One example is MgCl2, a coagulant used in the preparation of tofu from soybeans. For binary ionic compounds, the subscripts in the empirical formula can also be obtained by crossing charges: use the absolute value of the charge on one ion as the subscript for the other ion. This method is shown schematically as follows:
Crossing charges. One method for obtaining subscripts in the empirical formula is by crossing charges.
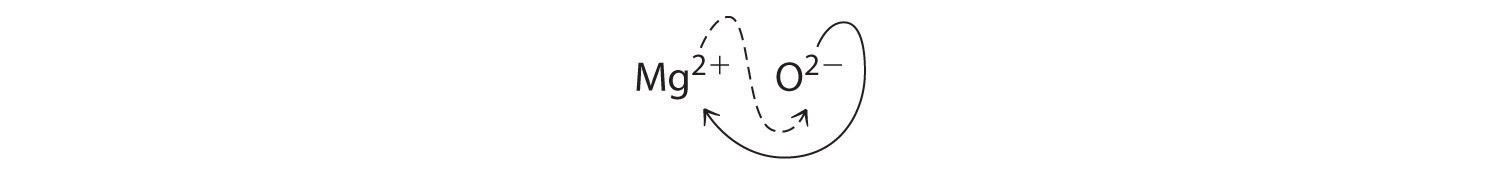
When crossing charges, it is sometimes necessary to reduce the subscripts to their simplest ratio to write the empirical formula. Consider, for example, the compound formed by Mg2+ and O2−. Using the absolute values of the charges on the ions as subscripts gives the formula Mg2O2:
This simplifies to its correct empirical formula MgO. The empirical formula has one Mg2+ ion and one O2− ion.
Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair.
- Ga3+ and As3−
- Eu3+ and O2−
- calcium and chlorine
Given: ions or elements
Asked for: empirical formula for binary ionic compound
Strategy:
A If not given, determine the ionic charges based on the location of the elements in the periodic table.
B Use the absolute value of the charge on each ion as the subscript for the other ion. Reduce the subscripts to the lowest numbers
to write the empirical formula. Check to make sure the empirical formula is electrically neutral.
Solution
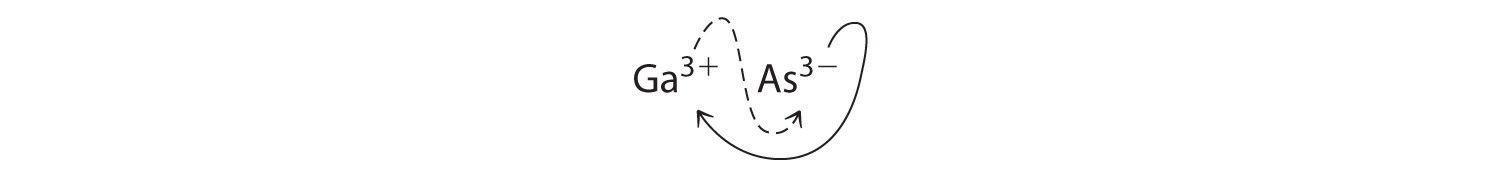
a. B Using the absolute values of the charges on the ions as the subscripts gives Ga3As3:
Reducing the subscripts to the smallest whole numbers gives the empirical formula GaAs, which is electrically neutral [+3 + (−3) = 0]. Alternatively, we could recognize that Ga3+ and As3− have charges of equal magnitude but opposite signs. One Ga3+ ion balances the charge on one As3− ion, and a 1:1 compound will have no net charge. Because we write subscripts only if the number is greater than 1, the empirical formula is GaAs. GaAs is gallium arsenide, which is widely used in the electronics industry in transistors and other devices.
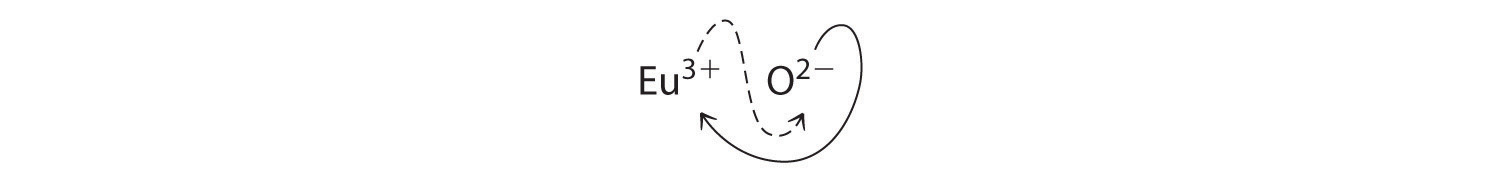
b. B Because Eu3+ has a charge of +3 and O2− has a charge of −2, a 1:1 compound would have a net charge of +1. We must therefore find multiples of the charges that cancel. We cross charges, using the absolute value of the charge on one ion as the subscript for the other ion:
The subscript for Eu3+ is 2 (from O2−), and the subscript for O2− is 3 (from Eu3+), giving Eu2O3; the subscripts cannot be reduced further. The empirical formula contains a positive charge of 2(+3) = +6 and a negative charge of 3(−2) = −6, for a net charge of 0. The compound Eu2O3 is neutral. Europium oxide is responsible for the red color in television and computer screens.
c. A Because the charges on the ions are not given, we must first determine the charges expected for the most common ions derived from calcium and chlorine. Calcium lies in group 2, so it should lose two electrons to form Ca2+. Chlorine lies in group 17, so it should gain one electron to form Cl−.
B Two Cl− ions are needed to balance the charge on one Ca2+ ion, which leads to the empirical formula CaCl2. We could also cross charges, using the absolute value of the charge on Ca2+ as the subscript for Cl and the absolute value of the charge on Cl− as the subscript for Ca:
The subscripts in CaCl2 cannot be reduced further. The empirical formula is electrically neutral [+2 + 2(−1) = 0]. This compound is calcium chloride, one of the substances used as “salt” to melt ice on roads and sidewalks in winter.
Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair.
- Li+ and N3−
- Al3+ and O2−
- lithium and oxygen
Answer:
- Li3N
- Al2O3
- Li2O
Polyatomic Ions
Polyatomic ions are groups of atoms that bear net electrical charges, although the atoms in a polyatomic ion are held together by the same covalent bonds that hold atoms together in molecules. Just as there are many more kinds of molecules than simple elements, there are many more kinds of polyatomic ions than monatomic ions. Two examples of polyatomic cations are the ammonium (NH4+) and the methylammonium (CH3NH3+) ions. Polyatomic anions are much more numerous than polyatomic cations; some common examples are in Table \(\PageIndex{4}\).
| Formula | Name of Ion |
|---|---|
| NH4+ | ammonium |
| CH3NH3+ | methylammonium |
| OH− | hydroxide |
| O22− | peroxide |
| CN− | cyanide |
| SCN− | thiocyanate |
| NO2− | nitrite |
| NO3− | nitrate |
| CO32− | carbonate |
| HCO3− | hydrogen carbonate, or bicarbonate |
| SO32− | sulfite |
| SO42− | sulfate |
| HSO4− | hydrogen sulfate, or bisulfate |
| PO43− | phosphate |
| HPO42− | hydrogen phosphate |
| H2PO4− | dihydrogen phosphate |
| ClO− | hypochlorite |
| ClO2− | chlorite |
| ClO3− | chlorate |
| ClO4− | perchlorate |
| MnO4− | permanganate |
| CrO42− | chromate |
| Cr2O72− | dichromate |
| C2O42− | oxalate |
| HCO2− | formate |
| CH3CO2− | acetate |
| C6H5CO2− | benzoate |
The method used to predict the empirical formulas for ionic compounds that contain monatomic ions can also be used for compounds that contain polyatomic ions. The overall charge on the cations must balance the overall charge on the anions in the formula unit. Thus, K+ and NO3− ions combine in a 1:1 ratio to form KNO3 (potassium nitrate or saltpeter), a major ingredient in black gunpowder. Similarly, Ca2+ and SO42− form CaSO4 (calcium sulfate), which combines with varying amounts of water to form gypsum and plaster of Paris. The polyatomic ions NH4+ and NO3− form NH4NO3 (ammonium nitrate), a widely used fertilizer and, in the wrong hands, an explosive. One example of a compound in which the ions have charges of different magnitudes is calcium phosphate, which is composed of Ca2+ and PO43− ions; it is a major component of bones. The compound is electrically neutral because the ions combine in a ratio of three Ca2+ ions [3(+2) = +6] for every two ions [2(−3) = −6], giving an empirical formula of Ca3(PO4)2; the parentheses around PO4 in the empirical formula indicate that it is a polyatomic ion. Writing the formula for calcium phosphate as Ca3P2O8 gives the correct number of each atom in the formula unit, but it obscures the fact that the compound contains readily identifiable PO43− ions.
Write the empirical formula for the compound formed from each ion pair.
- Na+ and HPO42−
- potassium cation and cyanide anion
- calcium cation and hypochlorite anion
Given: ions
Asked for: empirical formula for ionic compound
Strategy:
A If it is not given, determine the charge on a monatomic ion from its location in the periodic table. Use Table \(\PageIndex{4}\) "Common Polyatomic Ions and Their Names" to find the charge on a polyatomic ion.
B Use the absolute value of the charge on each ion as the subscript for the other ion. Reduce the subscripts to the smallest whole numbers when writing the empirical formula.
Solution:
a. B Because HPO42− has a charge of −2 and Na+ has a charge of +1, the empirical formula requires two Na+ ions to balance the charge of the polyatomic ion, giving Na2HPO4. The subscripts are reduced to the lowest numbers, so the empirical formula is Na2HPO4. This compound is sodium hydrogen phosphate, which is used to provide texture in processed cheese, puddings, and instant breakfasts.
b. A The potassium cation is K+, and the cyanide anion is CN−. B Because the magnitude of the charge on each ion is the same, the empirical formula is KCN. Potassium cyanide is highly toxic, and at one time it was used as rat poison. This use has been discontinued, however, because too many people were being poisoned accidentally.
c. A The calcium cation is Ca2+, and the hypochlorite anion is ClO−. B Two ClO− ions are needed to balance the charge on one Ca2+ ion, giving Ca(ClO)2. The subscripts cannot be reduced further, so the empirical formula is Ca(ClO)2. This is calcium hypochlorite, the “chlorine” used to purify water in swimming pools.
Write the empirical formula for the compound formed from each ion pair.
- Ca2+ and H2PO4−
- sodium cation and bicarbonate anion
- ammonium cation and sulfate anion
Answer:
- Ca(H2PO4)2: calcium dihydrogen phosphate is one of the ingredients in baking powder.
- NaHCO3: sodium bicarbonate is found in antacids and baking powder; in pure form, it is sold as baking soda.
- (NH4)2SO4: ammonium sulfate is a common source of nitrogen in fertilizers.
Summary
- There are two fundamentally different kinds of chemical bonds (covalent and ionic) that cause substances to have very different properties.
- The composition of a compound is represented by an empirical or molecular formula, each consisting of at least one formula unit.Contributors
The atoms in chemical compounds are held together by attractive electrostatic interactions known as chemical bonds. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. The ions are held together in a regular spatial arrangement by electrostatic forces. Most covalent compounds consist of molecules, groups of atoms in which one or more pairs of electrons are shared by at least two atoms to form a covalent bond. The atoms in molecules are held together by the electrostatic attraction between the positively charged nuclei of the bonded atoms and the negatively charged electrons shared by the nuclei. The molecular formula of a covalent compound gives the types and numbers of atoms present. Compounds that contain predominantly carbon and hydrogen are called organic compounds, whereas compounds that consist primarily of elements other than carbon and hydrogen are inorganic compounds. Diatomic molecules contain two atoms, and polyatomic molecules contain more than two. A structural formula indicates the composition and approximate structure and shape of a molecule. Single bonds, double bonds, and triple bonds are covalent bonds in which one, two, and three pairs of electrons, respectively, are shared between two bonded atoms. Atoms or groups of atoms that possess a net electrical charge are called ions; they can have either a positive charge (cations) or a negative charge (anions). Ions can consist of one atom (monatomic ions) or several (polyatomic ions). The charges on monatomic ions of most main group elements can be predicted from the location of the element in the periodic table. Ionic compounds usually form hard crystalline solids with high melting points. Covalent molecular compounds, in contrast, consist of discrete molecules held together by weak intermolecular forces and can be gases, liquids, or solids at room temperature and pressure.
An empirical formula gives the relative numbers of atoms of the elements in a compound, reduced to the lowest whole numbers. The formula unit is the absolute grouping represented by the empirical formula of a compound, either ionic or covalent. Empirical formulas are particularly useful for describing the composition of ionic compounds, which do not contain readily identifiable molecules. Some ionic compounds occur as hydrates, which contain specific ratios of loosely bound water molecules called waters of hydration.