3.24: Quiz 4B Key
- Page ID
- 19323
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Clearly indicate true (T) or false (F) for the following statements (2 pts each):
| __F__ | [CH3O]– is a stronger base than [CH3NH]– |
| __F__ | A Lewis acid is a compound that donates a pair of electrons. |
| __T__ | The higher the energy of activation, the more slowly the reaction will take place. |
| __F__ | A more stable chemical species is higher in energy. |
- Give the best answer for the following multiple choice questions: (2 pts each)
Which of the following molecules will have a higher boiling point?

Which of the following compounds is not a nucleophile?
 CH3ONa
CH3ONa- BF3
- NH3
- CH3OH
Which of the following is the weakest base?

Which of the following is incorrect?
- Delocalized electrons destabilize a compound
- Resonance stabilization is the extra stability a compound gains from having delocalized electrons
- When there are a greater number of relatively stable resonance contributors, then a molecule will have greater resonance stabilization
- both A and C
- both B and C
- (6 pts) Acrylamide is a potential carcinogen that can occur when starchy foods (such as potatoes) are fried or roasted. Compare the structures of acrylamide and allylamine. Circle the compound that has a more nucleophilic nitrogen and explain your answer.
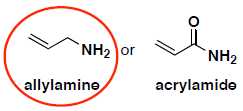
The nitrogen atom on acrylamide has delocalized electrons, so they are less available to react or attack an electrophile. The nitrogen atom on allylmine does not have delocalized electrons, so they are more available to react.
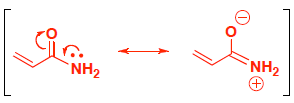
Resonance contributors show delocalized electrons, make the molecule more stable and the electrons on N are less available because they are shared.
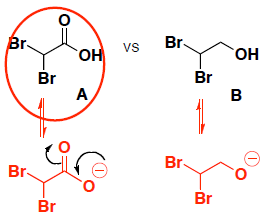
- (8 pts) Which compound (A or B) is a stronger acid? Provide the following information:
- draw the conjugate base (deprotonated form) of EACH molecule given
- circle the original acidic molecule that is more acidic (has the lowest pKa)
- provide a brief explanation for your choice
-
Both molecules have resonance stabilization, so we look at inductive effects. (the anions are both on oxygen, so there is not a size effect.)
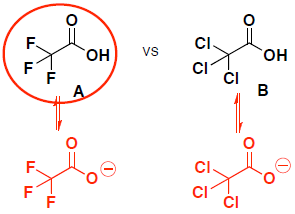
molecule A is a stronger acid because it has a stronger inductive effect (thru sigma bonds) from the electronegative F groups to stabilize the conjugate base. F is more electronegative than Cl.
compare stability of conjugate bases (must have negative charge on oxygen to show that the proton was removed)
molecule A is a stronger acid because it has both resonance stabilization (delocalized electrons thru pi bond) and also an inductive effect (thru sigma bonds) from the electronegative Br groups to stabilize the conjugate base.
molecule B only has inductive effects, so the conjugate base is less stable.
(Note, in general resonance thru pi bonds is stronger stabilizing than inductive effects thru sigma bonds)
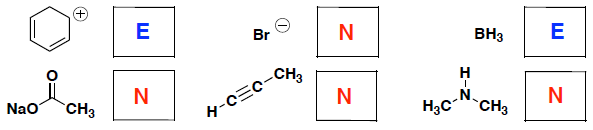
- (6 pts) Indicate whether each of the following molecules is more likely to behave as a nucleophile (N) or electrophile (E):
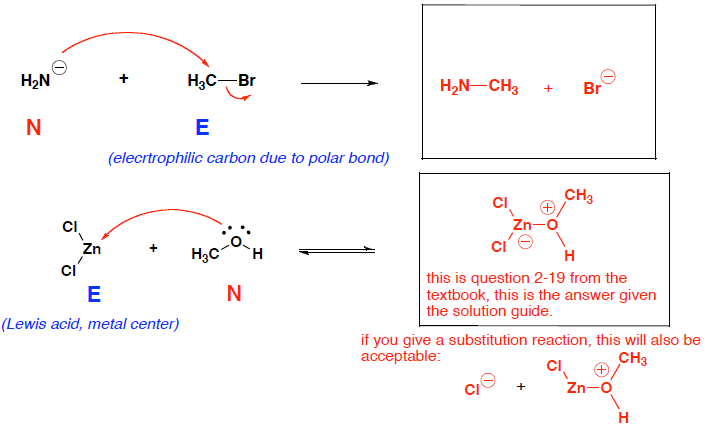
- (8 pts) Clearly label the nucleophile (N) and electrophile (E) for each reaction. Then write the product(s) of the following reaction, and show curved arrows for the movement of electrons. Donʼt forget to include formal charges in the product(s)!
- Extra Practice: (4 pts) Vitamin C, also known as ascorbic acid, has an acidic proton. Indicate which of the following protons is most acidic, and provide an explanation that includes structures to support your answer. (you only get points for your explanation, not guessing which proton…)

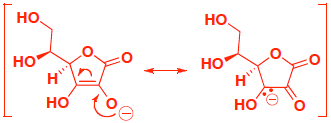
If you remove proton A, the conjugate base is stabilized by resonance with a negative charge shared on carbon
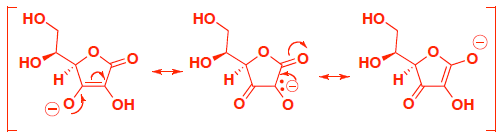
If you remove proton B, the conjugate base is stabilized by a total of three resonance contributors with a negative charge shared on carbon and two oxygens. you can draw arrows directly to delocalize on oxygen, since this is the most stable resonance contributor, because O is more EN than carbon