4.7: Experimental modes and Data Analysis
- Page ID
- 367413
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Designing the experiment requires an understanding of the four primary detection methods in X-ray Absorption Spectroscopies (XAS): (1) Transmission, (2) Fluorescence, (3) "Auger" Electron yield and (4) Total Electron Yield.
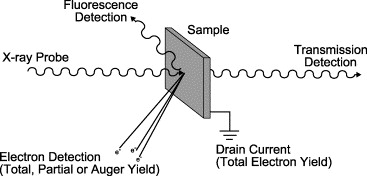
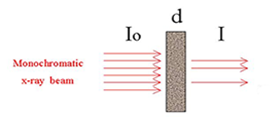
Transmission Mode
Absorption Length = distance over which x-ray intensity sets the fundamental length scale for choosing sample thickness, particle size, and sample homogeneity. This is often about 10 microns but varies depending on density and element that is probed. Sample cannot be significantly larger or it absorbs all the x-ray light.
The XAS technique is insensitive and higher concentrations of metal will provide higher quality data (up to around 5 mM or so). Sample cuvets are commonly made out of machined polycarbonate or Lucite with one X-ray transparent (e.g., Mylar) window. Minimum volumes useful on current generation beamlines are dictated by the cross section of the beam and usually one needs at least 50 microliters. One major limitation to the use of this technique is its inability to distinguish multiple site structures within a given sample. If the sample has heterogeneous metal environments, the resulting XAS-derived structure would simply be the weighted sum of all the structures contained in the sample. Thus, many EXAFS reports describe an ”average” structure. It is usually helpful to have some independent measurement that suggests that all of the metal being probed exists in a homogeneous type of site.
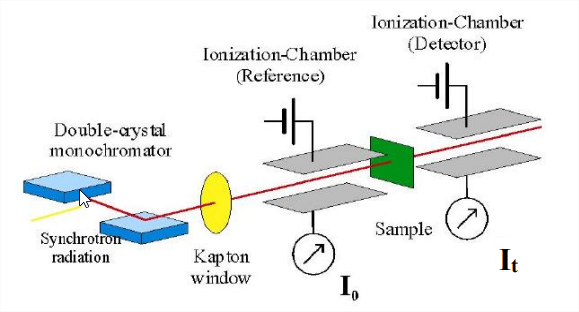
This schematic of an X-ray absorption spectrometer shows that the essential components are analogous to those required for any spectroscopic technique. However, operating in the X-ray region of the electromagnetic spectrum requires that each component be designed differently than their analogs in the UV, visible, or IR regions.
X-ray detectors are either gas ionization chambers (X-rays ionize an inert gas and charged plates collect and record the microcurrent generated) or fluorescence photon-counting detectors (scintillation counters, solid-state detectors). X-ray monochromators are two parallel single crystals of silicon (usually) that take advantage of Bragg diffraction to monochromate the Xrays. Tuning occurs by simply rotating the crystals to different incident angles. A light bulb is not a good source of X-rays, so instead all biological XAS is performed using synchrotron radiation generated by a storage ring.
You measure absorption just like in UV-VIS and IR (dispersive):
\[\frac{I}{I_{0}}=e^{-\mu(E) x} \nonumber \]
which can be converted to
\[\mu(x) x=\ln \left(\frac{I}{I_{0}}\right) \nonumber \]
Fluorescence Mode
X-ray fluorescence or XRF is the emission of characteristic "secondary" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays. X-ray fluorescence. If the electron has enough energy it can knock an orbital electron out of the inner shell of a metal atom, and as a result electrons from higher energy levels then fill up the vacancy and X-ray photons are emitted. This process produces a discrete spectrum of X-ray frequencies, called spectral lines. The spectral lines generated depends on the target (anode) element used and thus are called characteristic lines. Usually these are transitions from upper shells into K shell (called K lines), into L shell (called L lines) and so on.
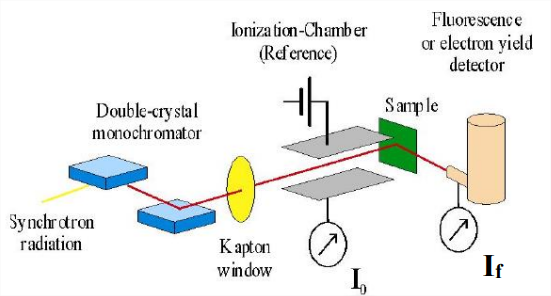
"Auger" Electrons
When an electron is removed from a core level of an atom, leaving a vacancy, an electron from a higher energy level may fall into the vacancy, resulting in a release of energy. Although sometimes this energy is released in the form of an emitted photon (XRF), the energy can also be transferred to another electron, which is ejected from the atom. This second ejected electron is called an Auger electron.
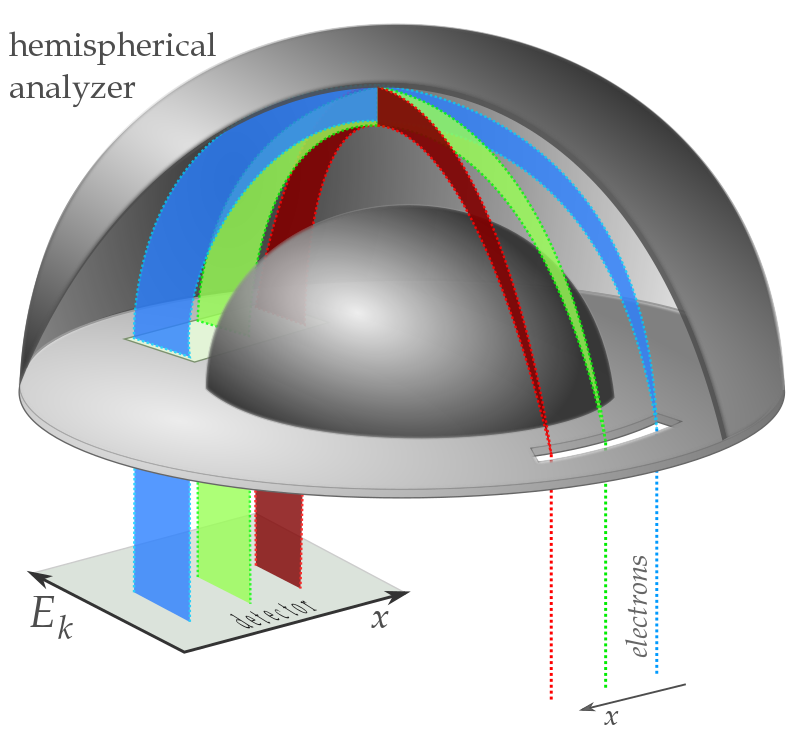
\(I \propto I_{0} \varepsilon \omega \Omega x \mu\)
with
- \(\mu\)-absorption coefficient
- \(x\)-layer thickness
- \(\omega\)-radiative or nonradiative yield
- \(\Omega\)-solid angle of collection
- \(\varepsilon\)-detection efficiency
Upon ejection the kinetic energy of the Auger electron corresponds to the difference between the energy of the initial electronic transition and the ionization energy for the electron shell from which the Auger electron was ejected. These energy levels depend on the type of atom and the chemical environment in which the atom was located.
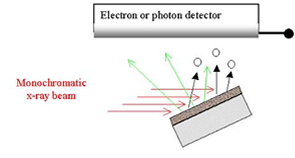
Total Electron yield
In the total electron yield detection mode, a solid is illuminated with a monochromatic beam of x-rays. Core electrons are excited into the conduction band of the solid, and the resulting current measured in the variant known as total electron yield. The intensity of the emitted primary Auger electrons is a direct measure of the x- ray absorption process are highly surface sensitive, similar to XPS. As they leave the sample, the primary Auger electrons create scattered secondary electrons (Figure \(\PageIndex{4}\)) which dominate the total electron yield (TEY) intensity. The TEY cascade involves several scattering events and originates from an average depth, the electron sampling depth L.
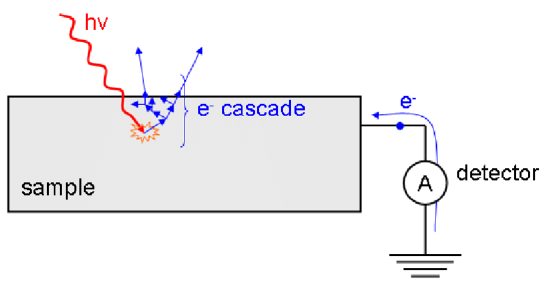
This detection scheme has significantly limitations for non-conducting samples.

