4.6: X-ray Absorption Spectroscopies
- Page ID
- 367412
Originally discovered by Wilhelm Roentgen in the nineteenth century, X-rays have become one of the most useful applications of spectroscopy in both science and medicine. X-ray spectroscopy is a common analytical technique with a broad range of applications, particularly in determining crystal structure and elemental analysis of solid samples. X-rays are a form of electromagnetic radiation with a much higher degree of energy than UV radiation. This extra energy allows X-rays to be absorbed by core electrons within atoms. Also, X-rays can penetrate crystal structures more than other forms of EM radiation, having a wavelength on the same order of magnitude as interatomic distances. This allows the X-rays to be diffracted, producing diffraction patterns of the crystal. The most common means of generating X-ray for these analyses is with an X-ray Tube, but a synchrotron can work also.The electromagnetic radiation resulting from inner orbital electron transitions or deceleration of high-energy electrons is referred to as X-rays. The absorption, diffraction, emission, fluorescence and scattering of X-rays is exploited in a variety of X-Ray spectroscopic techniques. There are three basic applications of X-ray analysis (Figure \(\PageIndex{1}\)):
- Diffraction of X-rays on crystalline materials to obtain their crystal structure (X-ray Diffraction (XRD)),
- Measurement of the energy of emitted X-rays during SEM imaging (X-ray Fluorescence (XRF) or x-ray emission spectroscopy (XES)) to give elemental information from the sample surface,
- Using X-rays to knock out core electrons of atoms to provide surface chemical information from samples (X-ray Photoelectron Spectroscopy (XPS, also known as ESCA))
These techniques reveal useful information about the structure and composition of matter. The three main techniques that we are focusing on are X-ray Fluorescence (XRF), X-ray Photoelectron Spectroscopy (XPS or ESCA) and Auger, Electron Spectroscopy (AES). The subtle differences between these three techniques are shown below.
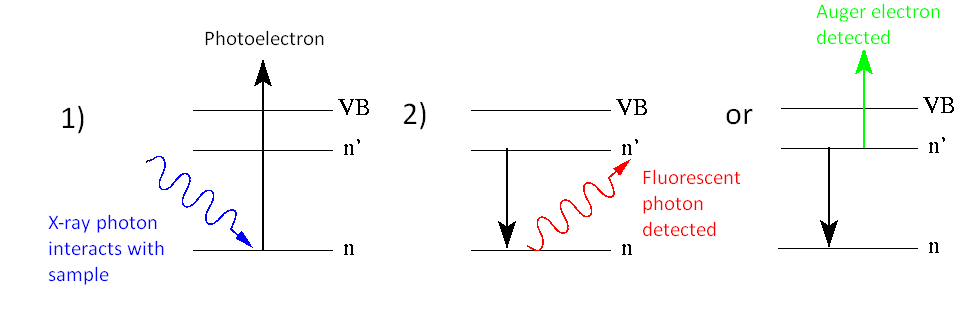
X-ray Photoelectron Spectroscopy (XPS)
XPS was discussed in the previous section and is in many ways the complete reverse of XRF in that an incoming X-ray photon causes the removal of a core or valence electron, although core electrons are more easily removed. These escaping electrons have a kinetic energy which is determined by the original photon and the binding energy of the atom. We can measure the kinetic energy of the electron and find out how tightly the electron was held in the atom. This is then used to find such information as oxidation state and bonding information. Like all X-ray techniques however, the information relates only to the top 100 Angstroms of the sample surface, making it useful for surface science techniques.
Auger Electron Spectroscopy
One of the two principal processes for the relaxation of an inner-shell electron vacancy in an excited or ionized atom. The Auger effect is a two-electron process in which an electron makes a discrete transition from a less bound shell to the vacant, but more tightly bound, electron shell. The energy gained in this process is transferred, via the electrostatic interaction, to another bound electron which then escapes from the atom. This outgoing electron is referred to as an Auger electron and is labeled by letters corresponding to the atomic shells involved in the process. For example, a KLILIII Auger electron corresponds to a process in which an LI electron makes a transition to the K shell and the energy is transferred to an LIII electron (illus. a). By the conservation of energy, the Auger electron kinetic energy E is given by
\[ E = E(K) − E(L_I) − E(L_{III}) \nonumber \]
where \(E(K,L)\) is the binding energy of the various electron shells. Since the energy levels of atoms are discrete and well understood, the Auger energy is a signature of the emitting atom.
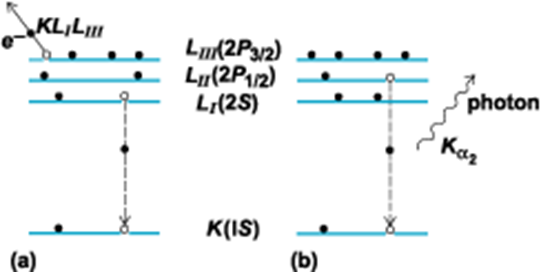
Auger spectroscopy is complimentary with XPS and most instruments allow both types of measurement to be made on a sample. The Auger process occurs in excited atoms which have a core electron removed. As in XRF, an upper electron drops to fill the vacancy, although instead of emitting a photon to release the excess energy, it transfers its energy to a neighboring electron which is then emitted from the atom. Auger electrons are of very low energy and are only emitted from the extreme surface of the sample. This is exploited by etching the surface of the sample with an ion beam, exposing progressively deeper layers into the sample. An electron beam is then directed onto the exposed regions. The Auger electrons produced by the beam come from each new layer exposed, allowing depth profiling to be achieved.
X-ray Fluorescence (XFS) or X-ray emission spectroscopy (XES)
X-ray Fluorescence (XFS) like other fluorescent techniques culminate with the emission of a photon. An atom which is induced into an excited state, usually by an electron beam causing the removal of a core electron from the atom, causes relaxation of the excited state. This relaxation occurs by an electron in a higher energy state dropping to fill the core level vacancy. The large energy decrease required to allow this to occur produces the release of an X-ray photon.
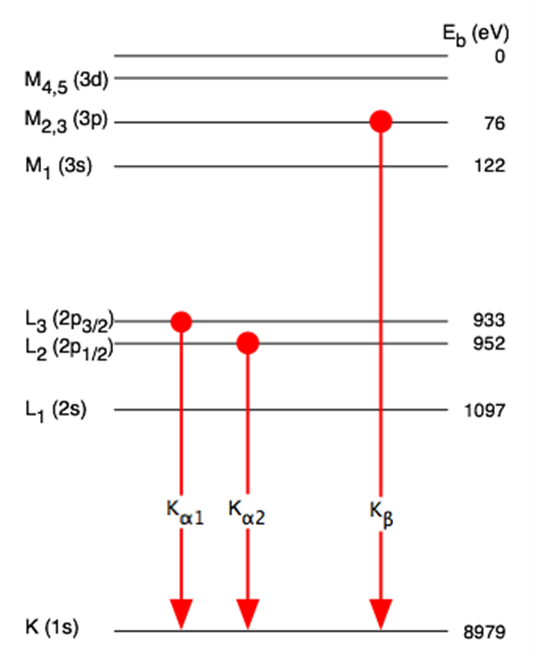
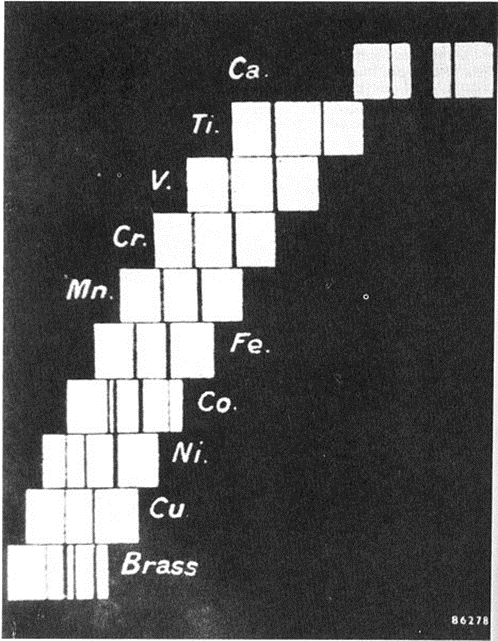

The relation between the atomic number Z of an element and the energy of its K and L x-ray absorption edges (Moseley law).

