2.11: Term Symbols
- Page ID
- 366112
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Term symbols are a shorthand method used to describe the energy, angular momentum, and spin multiplicity of an atom in any particular state. From a spectroscopic perspective, we need to know the values for the various types of angular momenta. Term symbols provide three pieces of information
- Total orbital angular momentum, \(L\)
- Multiplicity of the term, \(2S+1\)
- Total angular momentum, \(J\)
\[^{2S+1}L_J \nonumber \]
Total Angular Momentum
\[L=l_1+ l_2+ l_3+... \nonumber \]
- Maximum L is \(l_1+l_2\)
- Minimum L is \(|l_1-l_2|\)
\begin{array}{llllll}
\text { L: } & 0 & 1 & 2 & 3 & 4 \\
& \text { S } & \text { P } & \text { S } & \text { F } & \text { G }
\end{array}
Spin Multiplicity
We can also determine the multiplicity options using a Clebsch-Gordan series
\[S= ½+ ½, ½-½ \nonumber \]
For two electron system S=0 and the spin multiplicity given by
\[2S + 1 \nonumber \]
- For \(S=1\) (two unpaired electrons): \(2S+1 = 3\) This is called a triplet state
- For \(S=0\) (no unpaired electrons): \(2S+1 = 1\) This is called a singlet state
Total Angular Momentum
Permitted values of \(J\) again, given by a Clebsch-Gordon series
\[J= L + S, L + S-1, L + S-2 …. |L-S| \nonumber \]
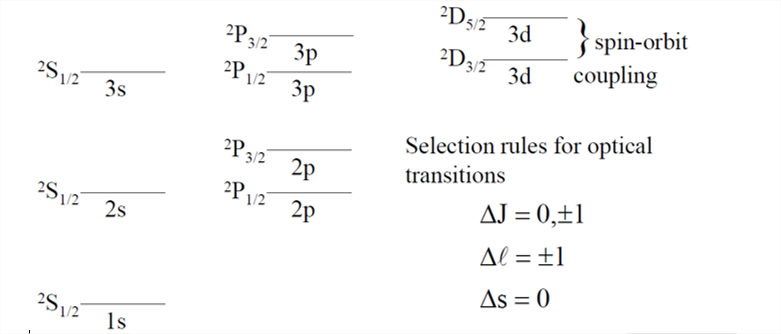
- H(1s1) : ground state term symbol is 2S1/2 . Hence only one possible state for this "configuration"
- He(1s2) : Ground state term symbol is 1S0. Hence only one state possible for this "configuration"
- Now, what about the excited hydrogen atom. For case with L =1, S = ½ J = 1 + ½ or 1 - ½ the term symbols are 2P3/2 and 2P1/2
A portion of the hydrogen atom transition level diagram for optical spectra then, will look like
And with allowed transitions:
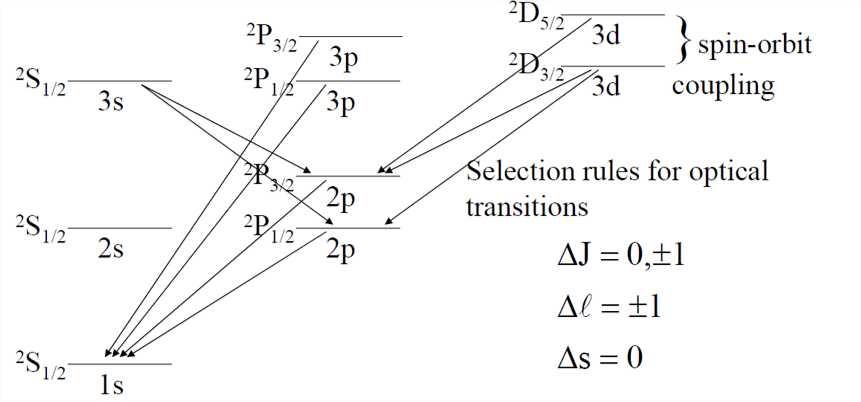
What about Ne? For 2p6 configuration, only one set of possible values.
\[M_L= m_1+ m_2+ m-3+ m-4+ m_5+ m_6 = 1 + 1 + 0 + 0 + (-1) + (-1) = 0 \nonumber \]
And in this case we also have
S = |Ms| = ms1+ ms2+ ms3+ ms4+ ms5+ ms6
= ½-½+ ½-½+ ½-½= 0
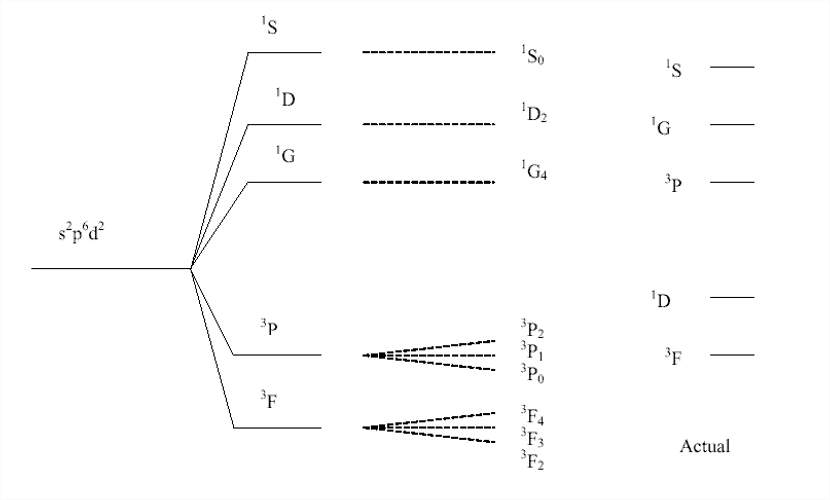
Thus, term is 1So. We will find this to be true for ANY filled subshell. More complicated terms occur with unfilled shells (e.g., excited states). How to dictate energy levels of these states? Use Hund's rules:
1. State with the largest value of S is most stable and stability decreases with decreasing S.
2. For states with same values of S, the state with the largest value of L is the most stable.
3. If states have same values of L and S then, for a subshell that is less than half filled, state with smallest J is most stable; for subshells that are more than half filled, state with largest value of J is most stable.
Example: Consider the terms 3D, 3P, 3S, 1D, 1P, 1S to describe the same electron distribution in an atom. In terms of stability we can rank these terms as:
1S > 1P > 1D > 3S > 3P > 3D Most stable
Given that the 3D states are most stable, which of these terms correspond to the most stable state? Since the two p subshells are less than half filled, we would predict that the 3D1term corresponds to the most stable state! Simple approach for finding the ground state term symbol for any atom:
- Find maximum value of S consistent with the Pauli Exclusion Principle: S = Smax.
- For S = Smax, find the maximum value of L consistent with the Pauli Exclusion Principle: L = Lmax.
- Apply Hund’s Rules to find J for most stable state.
He(1s12s1): An Excited State Configuration
Terms: 1S0 , 3S1
{ There is no 3S0 nor 3S-1 Term }