3.4: Aqueous Reactions
- Page ID
- 51202
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Outline the differences between strong electrolyte, weak electrolyte, and a nonelectrolyte
- Predict the solubility of ionic compounds in water using solubility rules
- Memorize seven strong acids that are strong electrolytes
- Distinguish ways of writing aqueous reaction equations
- General (molecular) ionic
- Total (complete) ionic
- Net Ionic
The objective of this section is to predict what will happen for single and double replacement reactions that occur in aqueous solutions. Note, if in a double displacement reaction two solutions combine and form a solid, you have a precipitation reaction. Many texts treat this as a type of reaction, but we will treat it as a subset of the double displacement reactions.
Electrolytes
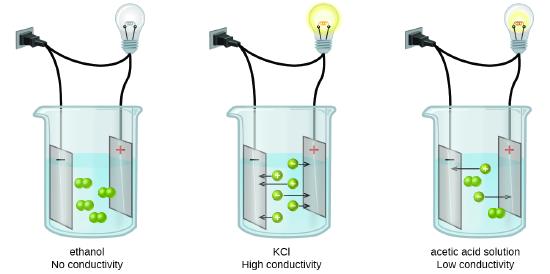
Pure water does not conduct electricity, and it has been observed that when a substance dissolves in water, it may produce mobile ions that allow the water to conduct electricity, and we call that compound an electrolyte, or it may not, in which case we call it a nonelectrolyte.
Exercise \(\PageIndex{1}\)
Both salt (NaCl) and table sugar, glucose (C6H12O6) dissolve in water. Why is salt water a strong electrolyte, while sugar water is a non-electrolyte?
- Answer
-
NaCl is an ionic compound that dissociates into ions that conduct electricity. C6H12O6 is a molecular compound that does not break up into ions, and therefore, does not conduct electricity. The sugar molecule remains intact, but each sugar molecule is separated from the other when added to water. The reason it dissolves in water is because of the term "the like dissolves the like", meaning both sugar and water are polar molecules. We will discuss this in more depth later in the text.
There are two basic ways an aqueous compound can be an electrolyte; being a soluble ionic compound or a strong acid.
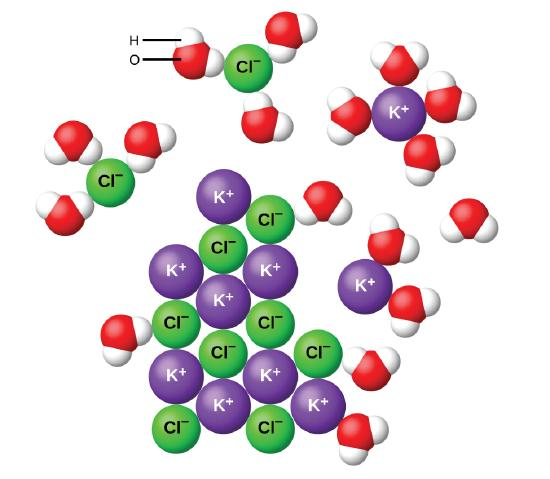
Soluble Ionic Compounds
- If ionic compounds dissolve and form a solution, the ions separate and are free to move about and conduct the electricity. But not all ionic compounds dissolve, and so they can be weak, strong or even nonelectrolytes. Typically, the nondissolved ionic compound forms a solid that falls to the bottom as a precipitate.
Covalent Compound that React with Water
Just as in the case with ionic compounds, covalent compounds can be weak, strong or nonelectrolytes. There are two types we will look at, acids and a type of bases called the amines.
Acids
The second way to produce an electrolyte happens when certain types of covalent molecules that react with the water. Acids give a proton to the water and so form ions as in the image below where HCl reacts with water to from chloride and hydronium ions (Figure \(\PageIndex{3}\)) .
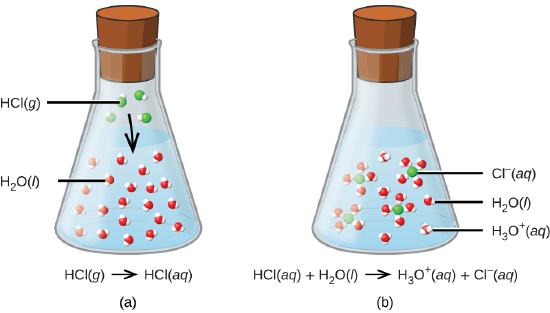
Strong acids are acids that completely react with water to form hydronium and the acid's anion.
Note
You need to memorize 7 strong acids, which are acids that completely react with water to form hydronium ions and the anion of the acid, and so these are strong electrolytes.
- 1. HCl, HBr, HI (heavier binary halides, HF is weak)
- 2. H2SO4 (sulfuric acid)
- 3. HNO3 (nitric acid)
- 4. HClO3 , HClO4 .
The above list is not comprehensive but in this class all other acids can be assumed weak unless they are called strong.
Amine Bases
Some bases are covalent compounds and the amines are an important group. They all have a nitrogen that can extract a proton from the water and form ions, as in the case below of ammonia, which grabs a proton from the water forming the weak electrolyte ammonium hydroxide (Figure \(\PageIndex{4}\)).
\[\text{NH}_{3} + \text{H}_{2}\text{O} \rightleftharpoons \text{NH}_{4}^{+} + \text{OH}^{-}\]
Types of Electrolytes
Compounds can be Strong, Weak, or Nonelectrolytes
- Strong Electrolytes - Strong Conductors of Electricity due to formation of a large number of Mobile Ions
- Weak Electrolytes -Weak Conductors of Electricity due to formation of a few Mobile Ions
- NonElectrolytes –Non Conductors of Electricity as they do not form Ions in aqueous solutions
Strong Electrolytes
Ionic - Soluble Salts and Strong Bases
NaCl(aq) --> Na+ (aq) + Cl- (aq)
NaOH(aq) --> Na+ (aq) + OH- (aq)
Covalent - Strong Acids (protonate water)
HCl(aq) + H2O --> H3O+ (aq) + Cl- (aq)
H2SO4 (aq) + H2O --> H3O+ (aq) + HSO4- (aq)
Weak Electrolytes
Ionic - Slightly Soluble Salts
CoCl2 (s) \( \rightleftharpoons \) Co+2(aq) + 2Cl- (aq)
Covalent - Weak Acids & Amine Bases (hydrolyze water)
HF(aq) + H2O \( \rightleftharpoons \) H3O+ (aq) + F- (aq)
NH3 (aq) + H2O \( \rightleftharpoons \) NH4+ (aq) + OH-
NonElectrolytes
Ionic - Insoluble Salts
CoS(aq) <=--> Co+2(aq) + S-2 (aq)
Covalent - Molecules which do not hydrolyze or protonate water
C12H22O11(s) + H2O --> C12H22O11(aq)
The following animations gives an atomic scale visualization of strong, weak and nonelectrolytes
Predicting Products for Double Displacement Reactions
Determine what the products will be for the following reactions. Initially we will ignore phases, but by the time we finish this exercise you will need to be able to identify phases as you write the equation. This process requires that you identify the ions in the reactants and for double displacement reactions swap partners and use the principle of charge neutrality to determine what the product are. For single replacement reaction you need to Figure which species (anion or cation) is gaining or losing charge and the other species is a spectator ion. Once that is done you need to balance the equations
Students frequently swap ions without thinking about the product formula and this leads to mistakes. It is suggested you follow these steps:
- Identify reactant ions and their charge
- Swap Ions
- Determine Product Formula based on principle of charge neutrality
- Balance Equation
Example:
\[Pb\left ( ClO \right )_{2}\left ( aq \right )+Na_{2}SO_{4}\left ( aq \right )\rightarrow\]
- Identify reactant ions and their charge

Table \(\PageIndex{1}\): Matrix showing the cations and ions for each of the two ionic compounds in the double displacement reaction
- Swap Ions
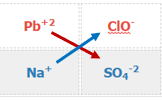
Table \(\PageIndex{2}\): Matrix of Table \(\PageIndex{1}\) showing the cations and ions swapping partners.
Pb+2 + SO4-2 \(\rightarrow\) ?
Na+ + ClO- \(\rightarrow\) ?
Note: If dealing with a strong acid, treat the "H" as a cation (break the acid HA into H+ and A-, where A is any anion, noting the number of hydrogens equals the charge of the anion, as the acid must be neutral). We will cover acids in the next section.
- Determine Product Formula based on principle of charge neutrality
\[Pb\left ( ClO \right )_{2}\left ( aq \right )+Na_{2}SO_{4}\left ( aq \right )\rightarrow PbSO_{4}\left ( ? \right )+NaClO\left ( ? \right )\]
If you need help determining formulas review section 2.6 - Balance Equation
\[Pb\left ( ClO \right )_{2}\left ( aq \right )+Na_{2}SO_{4}\left ( aq \right )\rightarrow PbSO_{4}\left ( ? \right )+2NaClO\left ( ? \right )\]
Exercise \(\PageIndex{2}\)
Predict products for the following reactions
- \(Mg_3\left ( PO_{4} \right )_2+NaClO_3\rightarrow ?\)
- \(AlPO_{4}+Na_2SO_3\rightarrow ?\)
- Answer a
-
\(Mg\left (ClO_{3} \right )_2+Na_3PO_4\)
- Answer b
-
\(Al_2 \left (SO_{3}\right)_3+Na_3PO_4\)
The next step is is figuring out the phase of the product. There are 3 common phases for aqueous reactions, solid (precipitate), aqueous and gas. In the case of aqueous products resulting from mixing two solutions, we state there is no reaction. In general chemistry 2 we will use mathematical formula of solubility products, but in this class we will use the Solubility rules, which are a good "rule of thumb" to determine if a salt will dissolve or not. Now note, these are all relative values, and even a soluble salt will form a precipitate, if you add a lot of it. For example, you can dissolve around 140 grams of potassium iodide in 100 g of water at 32oF, which makes it a very soluble salt, but a precipitate will occur if you try and add 200 g of KI to 100 g of water.
Solubility Rules
We saw that some compounds are soluble, other are partially soluble, and some are completely insoluble. How do we know determine this? We will start off with the simplest types, which are ionic compounds, and we will base these on the nature of the ions. There are sort of two opposing processes going on. Are the attractions of the ions in the crystal stronger than their attraction towards the water, or weaker? If the ionic attractions within the crystal are stronger, they do not dissolve and they form a precipitate. If on the other hand, the ions are more attracted to the water, they leave the crystal and the compound is soluble. We will use the solubility rules to determine if a salt is soluble or not.
What are the Solubility Rules?
These are what I am calling a "rule of thumb," and allow us to roughly predict if a salt will dissolve or not. It must be understood that the concept is relative, for example, table salt is considered a soluble salt and if you add table salt to water it will dissolve a lot, up to 359g per liter, but at that point it becomes saturated, and any more will form a precipitate. On the other hand silver chloride is an insoluble salt, and you can only dissolve 0.0019g into 1 liter, but any more will fall to the bottom as a precipitate.
Note, some textbooks give slightly different rules, and this set is incomplete. If your text is different, please discuss this with your instructor. When you get to general chemistry 2 you will learn a different approach, where we can quantify the amount dissolved for an insoluble salt, like the 1.9 mg/liter for silver chloride.
Is there a strategy to using the solubility rules?
Yes, for the ones I have set up below.
- We first look at the [+] cation, and ask if it is any one of the cations listed in step IA. If yes, we say it is soluble, and the question is answered. After this step we focus on the [-] anions. If it is not soluble from step 1A, we go to 1B, and if the anion is from this list, it is soluble.
- We now go to the compounds that are usually soluble, step II, and you need to memorize the exceptions, which are insoluble.
- We now go to the compounds that are usually insoluble, step III, and you need to memorize the exceptions, which are soluble.
Solubility Rules
|
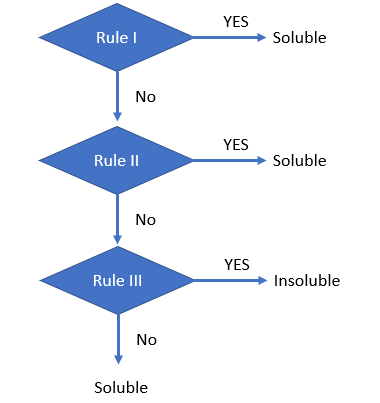 |
In the following video we will first predict the products for the double displacement reaction of aluminum sulfate and barium chloride, and then apply the solubility rules to determine the phases of the products.
Al2(SO4)3 + BaCl2 \(\rightarrow\) ?
Exercise \(\PageIndex{3}\)
Use the solubility rules to determine if the following compounds are soluble or insoluble. Indicate answer by writing formula followed by (aq) for soluble and (s) for insoluble. (You may also want to write the names of the species)
- PbSO4
- NaClO4
- Answer a
-
Insoluble. Sulfate ions are typically soluble, but lead(II) is an exception, so lead(II) sulfate is an insoluble solid. This means that PbSO4 (s) is not broken up into ions in water, but is a precipitate.
- Answer b
-
Soluble. perchlorate ions and alkali metals are soluble, so sodium perchlorate is soluble, meaning that it breaks up into ions, Na+(aq) + ClO-4 (aq) in water.
Exercise \(\PageIndex{4}\)
Identify the following as a strong electrolyte, a weak electrolyte, or a non-electroylte.
- Ba(NO3)2
- H3PO4
- C6H12O6
- HNO3
- AgBr
- Answer a
-
strong electrolyte - Rule 1B
- Answer b
-
weak electrolyte - weak acid (not one of the seven strong acids)
- Answer c
-
non-electrolyte - molecular compound, not ionic
- Answer d
-
strong electrolyte- strong acid (one of the seven strong acids)
- Answer e
-
non-electrolyte - insoluble precipitate - Exception to Rule 2A
Mixtures of Multiple Solutions
Consider 3 aqueous salt solutions each with different cations and anions. For example, consider NaCl, AgNO3 and (NH4)2CO3. There are 3 cations and 3 anions which results in 32 or 9 combinations (of which 3 are the reactants). This seems like a very complicated problem (4 salts would result in 16 potential combinations and 5 salts would result in 25) and so we need to develop a technique to see the problem. This can be done through a matrix, where the rows represent the cations, the columns the anions and the cells the potential combinations:
| Cl- | NO3- | CO3-2 | |
| Na+ | NaCl | ||
| Ag+ | AgNO3 | ||
| NH4+ | (NH4)2CO3 |
Now think about it for a minute. If you look at each row it starts with a cation, and if rule 1A says it is soluble, you can knock out the whole row, giving it XXX in table \(\PageIndex{5}\) to indicate no precipitate (all salts sodium and ammonium are solube), then then look at each column and according to rule 1B, you can knock out the middle column because all salts of nitrate are soluble. This reduced your workload to just two cells.
| Cl- | NO3- | CO3-2 | |
| Na+ | XXX | XXX | XXX |
| Ag+ | AgCl (s) | XXX | Ag2CO3(s) |
| NH4+ | XXX | XXX | XXX |
The following video shows how to solve these problems in the shortest amount of time for the following reaction:
NaCl + Na3PO4 + AgNO3 + Pb(NO3)2 + Na2SO4
Exercise \(\PageIndex{3}\)
Identify if any precipitates that would form if the following solutions are mixed:
- Pb(ClO4)2(aq), K2SO4(aq), AgCH3CO2 (aq) + KCl(aq)
- Ba(ClO3)2(aq), Li2SO4(aq), NH4CH3CO2 (aq) + BaCl2
- Answer a
-
ClO4- SO4- CH3CO2- Cl- Pb+2 XXX PbSO4 (s) XXX PbCl2 (s) K+ XXX XXX XXX XXX Ag+ XXX Ag2SO4 (aq) XXX AgCl (s) K+ XXX XXX XXX XXX So the answer is PbSO4 (s), PbCl2 (s) and AgCl (s), everything else is aqueous.
- Answer b
-
d
ClO4- SO4-2 CH3CO2- Cl- Ba+2 XXX BaSO4(s) XXX BaCl2(aq) Li+ XXX XXX XXX XXX NH4+ XXX XXX XXX XXX Ba+2 XXX BaSO4(s) XXX BaCl2(aq) So the answer is BaSO4 , everything else is aqueous.
Describing Aqueous Equations
There are three basic ways you will need to know that are used to describe aqueous chemical reactions. These are typically single and double displacement reactions. The difference between these lies in how you describe the ions of dissolved ionic compounds. Do you describe them as the separate ions, which are really what is floating around, or do you describe them by their neutral compound formula? That is, you can represent the solution as either the salt(aq) or its ions
\[NaCl(aq) \; \text{ means } \; Na^+(aq)+Cl^-(aq)\]
The General (Molecular) Equation uses the formula of the salt, the total ionic equations separates soluble salts (and strong acids) into their ions, while the net ionic equation cancels any ions that appear on both the reactant and product sides of the balanced equation, the "spectator ions", as they do not participate in the reaction. The net ionic equation typically describes the actual chemistry of the reaction, and what is going on.
- General (Molecular)Equation
- Total Ionic Equation
- Net Ionic Equation
In solving these problems remember, (aq) = aqueous, (s) = solid, (g) = gas and (l) = liquid, and be sure the final equation is balanced.
General (Molecular) Equation
The General or Molecular Equation describes the reactions in terms of the formula of the salts, indicating the phase. These are often (incorrectly) called "molecular equations" because they use the formula of the neutral compound as if it was a molecule. The following equation is an example of a general double displacement reaction, and given the reactants, you need to be able to predict product compounds, balance the equation and predict the phases.
\[Pb\left ( ClO_{4} \right )_{2}\left ( aq \right )+Na_{2}SO_{4}\left ( aq \right )\rightarrow PbSO_{4}\left ( s \right )+2NaClO_{4}\left ( aq \right )\]
As indicated above, the aqueous ions separate on dissolution, and so we could write them out as separate entities, which is the Total or Complete Ionic Equation.
Total Ionic Equation
The Total or complete ionic equation writes soluble ionic compounds in terms of their ions. So if it is an ionic compound with (aq), you break it into its ions. If it is covalent, you do not break it into ions. If it is an acid, you only break it into its ions if it is strong, and that will be covered in the next section, but weak acids that are dissolved, like HF(aq) do not break into ions, while strong ones like HCl do.
Describing eq. 3.4.6 in terms of a net ionic equation gives:
\[Pb^{+2}\left ( aq \right )+2ClO_{4}^{-}\left ( aq \right )+2Na^{+}\left ( aq \right )+SO_{4}^{-2}\left ( aq \right )\rightarrow PbSO_{4}\left ( s \right )+2Na^{+}\left ( aq \right )+2ClO_{4}^{-}\left ( aq \right )\]
Net Ionic Equation
The net ionic equation tells you what is happening, that is, what bonds (covalent or ionic) are being created. If you look at equation 3.4.7 you see there are two perchlorate and two sodium ions on both sides of the equation, and so we call them spectator ions because they did not react. If we cancel them the equation is still balanced and this gives the net ionic equation, which in this reaction results in the formation of the ionic compound, lead (II) sulfate.
\[Pb^{+2}\left ( aq \right )+SO_{4}^{-2}\left ( aq \right )\rightarrow PbSO_{4}\left ( s \right )\]
This is often called a precipitate reaction as the lead (II) sulfate crashes to the bottom of the container, and some textbooks classify precipitation reactions as a class of reaction.
Example \(\PageIndex{1}\)
Write the general, complete and net ionic equations for the reaction of barium chloride and potassium sulfate
Solution
\[\underbrace{BaCl_2(aq) + K_2SO_4(aq) \rightarrow BaSO_4(s) + 2KCl(aq)}_{\text{General (Molecular) Equation}} \nonumber \]
\[\underbrace{Ba^{+2}(aq)+\overbrace{2Cl^-(aq) +2K^+(aq)}^{\text{Spectator Ions}}+SO_4^{-2}(aq\rightarrow BaSO_4(s)+\overbrace{2Cl^-(aq) +2K^+(aq)}^{\text{Spectator Ions}}}_{\text{Complete Ionic Equation}} \nonumber\]
\[\underbrace{Ba^{+2}(aq)+SO_4^{-2}(aq\rightarrow BaSO_4(s)}_{\text{Net Ionic Equation}} \nonumber\]
Exercise \(\PageIndex{1}\)
Write balanced net ionic equations to describe any reaction which occur when the following solutions below are mixed.
- \(Na_2CO_3 + Sr(NO_3)_2 \)
- \(Cu_2SO_4 + BaCl_2 \)
- Answer a
- \[Sr^{2+}(aq) + CO_3^{-2} (aq) \rightarrow SrCO_3 (s) \nonumber\]
- Answer b
- \[Ba^{2+}(aq) + SO_4^{2-} (aq) -> Ba(SO_4) (s) \nonumber\]
Preci
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- November Palmer, Ronia Kattoum & Emily Chaote (UALR)


