9.2: Valence Bond Theory
- Page ID
- 158467
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
 |
UALR 1402: General Chemistry I |
 |
Introduction
Valence bond theory is an approach to bonding where molecular orbitals (wave functions) are considered to be the result of the mixing of pure atomic orbitals. For example, in the second period there are 4 orbitals, one 2s and three 2p, and for the hydrogen atom, the s orbital is lower in energy. When a second period atom forms two bonds, as in BeH2 , the beryllium has two bonding orbitals and they are identical in energy. So valence bond theory accounts for this by saying that one of the p orbitals mathematically mixed with the s orbital forming two new sp orbitals, each of which is 50% s in character and 50% p in character. This model gives 5 basic types of hybrid orbitals that account for molecules that have between 2 and 6 bonds to the central atom.
Five Basic Types of Hybrid Orbitals
sp 1/2s & 1/2p
sp2 1/3 s & 2/3 p
sp3 1/4 s & 3/4 p
sp3d 1/5 s & 3/5 p & 1/5 d
sp3d2 1/6 s & 3/6 p & 2/6d
Note, second period elements have no d orbitals and so can not have more than an octet of electrons, that is, only the third period and greater can form sp3d and sp3d2 orbitals.
Requirements for Orbital mixing
- Orbitals must be of similar energy.
- Orbitals must have wave functions in same region of space
- Remember wavefunctions can interact constructively or destructively, where in constructive overlap the wave functions add, and in destructive overlap they cancel each other out.
- Number of Hybrid Orbitals Equals the Number of Atomic Orbitals Mixed.
Bond Formation and \(\sigma\) bonds
One way of looking at the formation of a bond is for two atoms with a free radical atomic orbital (atomic orbital with one electron) to come together and overlap and form molecular orbitals. In reality, two orbitals form, one with constructive overlap (the bonding orbital) and one with destructive overlap (which we will see in the next section is the antibonding orbital). In Figure \(\PageIndex{1}\) we can visualize the creation of a molecular bonding orbital for hydrogen by the overlap of the two atomic 1s orbitals. This two electron orbital is called a Sigma (\(\sigma\)) bond.
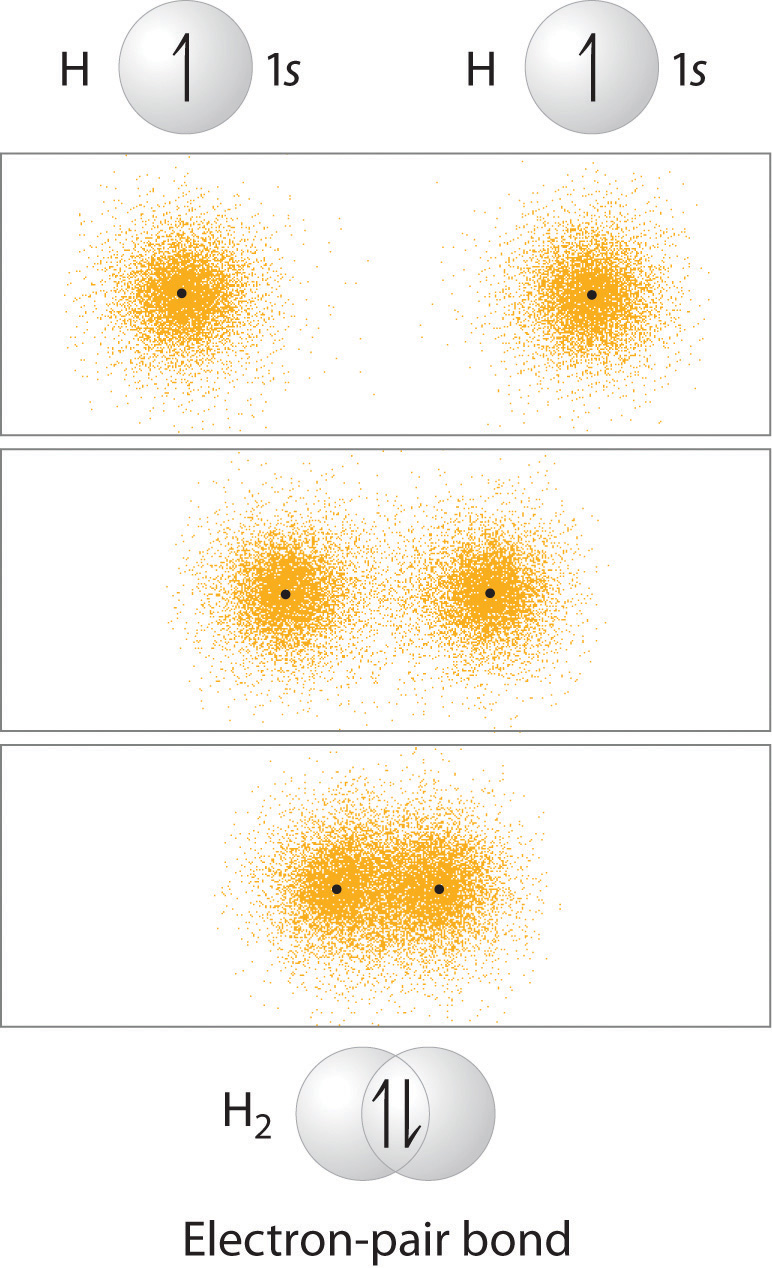
Figure \(\PageIndex{1}\): The two isolated hydrogen atoms at the top have one electron in their 1s orbital, and so they are free radicals. When they form a bond, each of the hydrogen donates one electron. This bond is called a \(\sigma\) bond because there is overlap along the internuclear axis.
In valence bond theory we will often visualize bonds being formed this way, where each atom donates one of the electrons. This means that to have 2 bonds, you need two free radicals, to have 3 bonds you would need 3, ....to have 6 bonds you would need 6 free radicals (6 orbitals with one electron each). Note, this is not always the case, and sometimes an orbital with two electrons on one atom can overlap with an empty orbital on another, which would be called a dative or coordinate covalent bond, but we will ignore those for now.
Overview of Orbital Hybridization
Video \(\PageIndex{1}\): (10:11 min, https://youtu.be/qraDpWX_msY ) Molecular Shape and Orbital Hybridization YouTube uploaded by Van Wyk (may not be original author).
sp hybrid orbital (linear electronic geometry)
The beryllium in beryllium hydride (BeH2) is an example of an sp hybrid. If we look at the ground state electron configuration of Be, it is [He]1s2, and so it has a filled valence orbital and as such, could not bond. This is shown on the left electron configuration of Figure \(\PageIndex{2}\), where on the right, the two red arrows are the Be sp orbitals which are equal in energy (half way between the 2s and sp) and with one electron in each, can overlap with the 1S orbitals of two different hydrogen atoms.

Figure \(\PageIndex{2}\): electron configuration showing that Be with a full orbital in its ground state must hybridize if it is to make two bonds.
Why is the sp hybrid linear?
In Figure \(\PageIndex{3}\) we note that the p orbitals align along Cartesian axes, and so if we consider the s to hybrize with the px then then the two sp orbitals face in opposite directions along the x-axis.
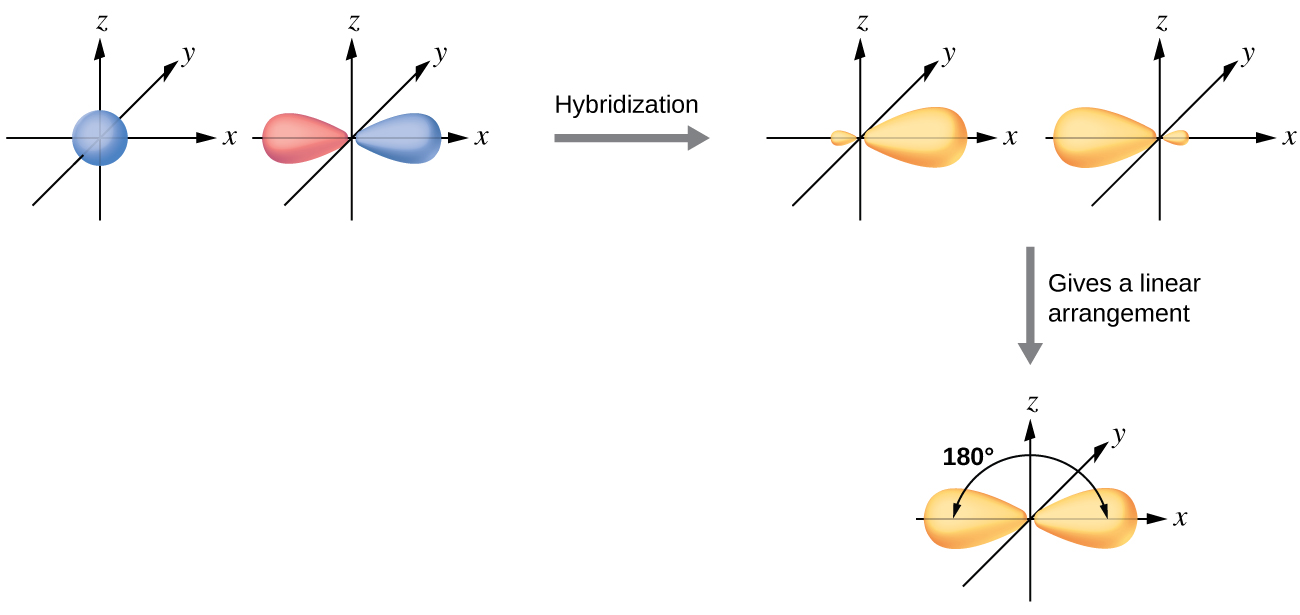
Figure \(\PageIndex{3}\): the S and p orbitals on the left hybridize to form two sp orbitals on the right. Note the p has a node at the nucleus while the S does not, and thus there is a minor lobe on each of the hybrids. The bottom image is showing the major lobe of both orbitals being aligned at 180 degrees.
Beryllium chloride forms a similar structure with the Be having a sp hybrid.

Figure \(\PageIndex{4}\): Beryllium is also sp hybridized in beryllium chloride.
Exercise \(\PageIndex{1}\)
What is the orbital of the chlorine in \(\ce{BeCl2}\)?
- Answer
-
it is a 3p orbital as chlorine has one free-radical orbital in it's valence state, and so there is no need to hybridize it.
sp2 hybrid orbital (trigonal planar electronic geometry)
The ground state of boron is [He]2s22p1 and so can not account for the three bonds in boron trifluoride, and the valence bond approach it to hybridize the 2s with two of the 2p orbitals giving three sp2 hybridized orbitals with one electron each, which can then bond with the lone electron in a fluorine 3p orbital, as indicated in Figure \(\PageIndex{5}\)

Figure \(\PageIndex{5}\): Boron ground state electron configuration on left, and the hybrid orbital configuration on the right that is involved with the three bonds in boron trifluoride.
Note the energy of the hybrid orbitals in Figure \(\PageIndex{5}\) is 2/3rds up from the s to the p, and if the 2px and 2py are hybridizing with the 2s orbital, the hybrid orbitals will be in the X-Y plane and according to VSEPR theory will take a trigonal planar orientation as described in Figure \(\PageIndex{6}\)

Figure \(\PageIndex{6}\): trigonal planar orientation of sp2 hybridized orbitals.
In Figure \(\PageIndex{7}\) the nitrogen and carbon atoms are sp2 hybridized. In the case of ClNO, the nitrogen has a lone pair in one of the hybrids and forms sigma bonds with the others. The second orbital of the N=O bond is a \(\pi\) bond and not form from a hybrid orbital, but from an unhybridized p orbital. Likewise, the carbon atoms of the other two structures have sp2 hybridized orbitals forming 3 sigma bonds each, with double bonds being a \(\sigma\) and \(pi\) bond. We will look at these in more detail in the next section.

Figure \(\PageIndex{7}\): The nitrogen and carbon atoms of these three molecules are sp2 hybridized orbitals.
sp3 hybrid orbital (tetrahedral electronic geometry)
Molecules with 4 "electron domains" have sp3 hybridized orbitals. The ground state of carbon is [He]2s22p2, which according to Hunds Rule has both of the 2p electrons in different orbitals wit the same spin (Figure \(\PageIndex{8}\)). Although it may not be clear, the energy is 3/4ths the way up between the s and the p orbital.

Figure \(\PageIndex{8}\): On the left is the unhybridized orbitals of carbon, which could only form 2 bonds. On the right are the 4 sp3 hybridized orbitals of carbon with it's 4 valence electrons in black, and the 4 1s valence electrons of 4 hydrogens in red, resulting in the 4 sigma bonds of methane (CH4).
The four sp3 hybridized orbitals result from the mixing of 1 s orbital with 3 p orbitals orient in 3D space as they involve a px, py & pz and according to VSEPR orient in a tetrahedral structure as shown in Figure \(\PageIndex{9}\)

Figure \(\PageIndex{9}\): Tetrahedral structure of the four sp3 hybridized orbitals.
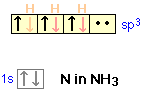
In the case of methane, all 4 hybrid orbitals have sigma bonds. In Figure \(\PageIndex{10}\) we see the electron configuration of nitrogen in ammonia and that there is one hybrid orbital that has a lone pair of electrons. This results in the tetrahedral electronic geometry arising from the sp3 hybridized orbitals, but a trigonal pyramidal molecular geometry.
 |
 |
Figure \(\PageIndex{10}\): Electron configuration of nitrogen in hybrid orbitals of ammonia results in the trigonal bipyramidal molecular geometry
In Figure \(\PageIndex{11}\) we see the electron configuration of oxygen in water and that there are two hybrid orbitals that has a lone pair of electrons. This results in the tetrahedral electronic geometry arising from the sp3 hybridized orbitals, but a bent 104.5 deg molecular geometry.
 |
  |
Figure \(\PageIndex{11}\): Electron configuration of water with oxygen's 6 electrons (black) in four sp3 hybridized orbitals, and 2 \(\sigma\) bonds arising from the two orbitals that only have one electron from oxygen (black) and one from hydrogen (red). As the lone pairs take up more space, the bond angle is less than the ideal 109.5 VSEPR bond angle.
Expanded Octets: sp3d & sp3d2 hybrid orbitalds
- Molecules with 5 "electron domains" have sp3d hybridized orbital
- Molecules with 6 "electron domains" have sp3d2 hybridized orbital
Figure \(\PageIndex{12}\) shows the orbitals being mixed to form sp3d and sp3d2 hybridized orbitals, respectively forming trigonal bipyrmaidial and octahedral electronic geometries.

Figure \(\PageIndex{12}\): formation of sp3d and sp3d2 hybride orbitals from the contributing atomic orbitals
Summary table of hybrid orbitals and VSEPR Shapes

Table \(\PageIndex{1}\): Summary of VSEPR electronic geometries and associated hybrid orbitals
Deeper look into Multiple Bonds Bonds
So far we have described single bonds as \(\sigma\) bonds, where the orbitals of two atoms overlap to form a bonding orbital with electron density along the internuclear axis. In this section we will describe multiple bonds which can be either double (1 \(\sigma\) and \(\pi\)) or a triple bond (2 \(\sigma\) and 2 \(\pi\)). A \(\pi\) bond occurs from the overlap of two unhybridized p orbitals on adjacent atoms that are perpendicular to the internuclear axis and aligned in the same plane. This is easiest to understand by looking at the formation of a double bond.
Double bonds
Ethylene C2H2 is the simplest molecule to have a carbon-carbon double bond, it is a planar molecule with both carbons sp2 hybridized.

Figure \(\PageIndex{13}\): Ethylene

Figure \(\PageIndex{13}\): Electron configuration of carbon as an isolated atom (left) and sp2 hybridized (right) as in ethylene.
Contributors
- Narration: Bob Belford
- Images from LibreTexts of Stepen Lower, OpenStax

