8.5 Exceptions to the Octet Rule
- Page ID
- 158459
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exceptions to the Octet Rule
The "octet rule" says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. We saw how we can predict the number of bonds and lone pairs of of electrons if atoms abide by the octet rule. However, there are many molecules that do not follow the octet rule. In fact we have already covered one, hydrogen, which can only have one bond, or two electrons. There are two types of exceptions, molecules with atoms having less than an octet, or molecules with atoms having more than an octet. There is also another class of exceptions, which are molecules that have odd numbers of electrons. In the next chapter we will cover molecular orbitals, which are different than the s,p & d atomic orbitals, and will be able to correlate the octet rule, and it's exceptions to the types of orbitals being occupied, and the number of electrons available to occupy those orbitals.
Atoms that can have a Reduced Octet
- Hydrogen: Hydrogen always forms one bond and so has a duet. The valence orbitals of hydrogen (and helium) have a principle quantum number of 1, and so there is only one orbital available, the 1s. Since each orbital can contain up to two electrons (of opposite spin), hydrogen can have 2 electrons in its Lewis Dot Structure. Note, there are cases like boron trihydride that have "bridging hydrogens", which are covalently bonded to two borons. These are very uncommon and in the class, hydrogen always has a duet (two electrons).
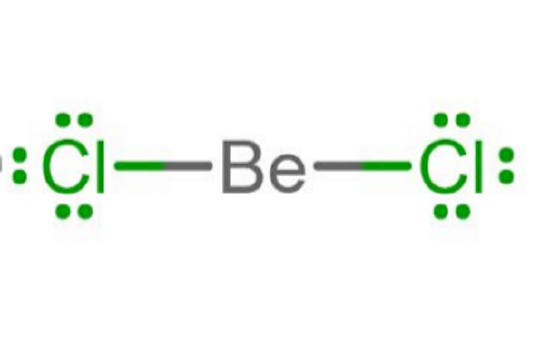
- Group IIA atoms like Beryllium often have two valence electrons. Because they only have two valence electrons to share, they will form two covalent bonds and will have no lone pairs, as in the case of BeH2 and BeCl2.
-
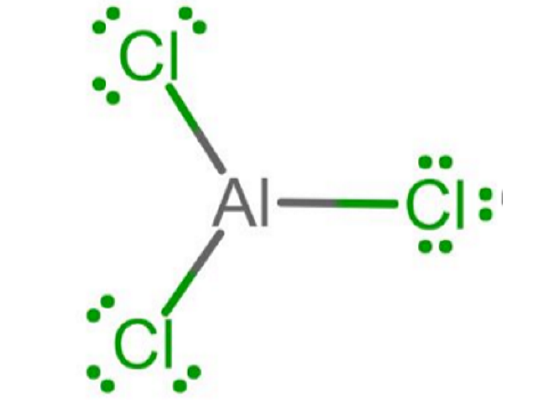
- Group IIIA atoms like aluminum will have only 3 valence electrons to share and will form three bonds without any lone pairs of electrons, as in the case of AlCl3.
-
Can atoms like Be and Al have form enough bonds to form a full octet. Yes, if another atom donates a lone pair (not just one electron) into the empty orbital, this can happen. We will
2. Atoms with an expanded octet
To have an expanded octet (more than 8 electrons) you need more than 4 orbitals. In the next chapter we will learn that molecular orbitals are different than atomic orbitals, but one way to describe molecular orbitals is by assuming they are the result of different atomic orbitals mixing with each other, and the number of molecular orbitals must equal the number of atomic orbitals that were mixed to create them. This means that you need to start using d-orbitals, and those are only available for atoms in the third period or below (So atoms in the third period or greater can have expanded octets. Phosphorous often has 5 orbitals (10 electrons) and sulfur often has 6 orbitals (12 electrons) because they are in the third period, but nitrogen and oxygen can never have expanded octets because they are in the second period and there is not such thing as a 2d orbital.
Why can't elements in the second row have an expanded octet?
For the second row, the principle quantum number is 2, and so you have s and p orbitals in the valence shell. As there is one type of S, (2s) and three types of P, (px, py & pz), there are only 4 available orbitals, which can take up to 8 electrons (an octet). NOTE: in the next chapter we will study molecular orbitals, and the orbitals in molecules are not atomic orbitals (s,p,d,f), but are different. That said, the number of molecular orbitals is determined by the number of atomic orbitals in the valence shell, but they are different.

