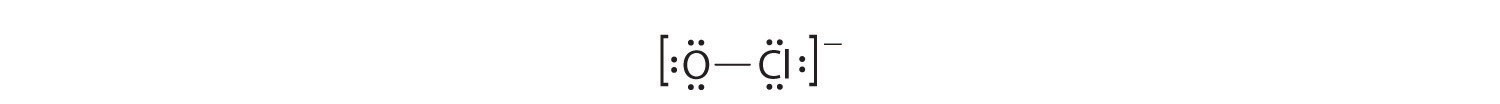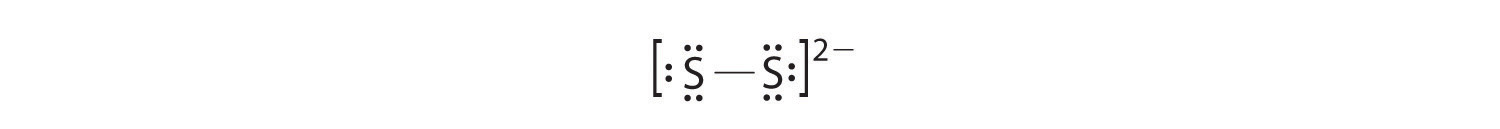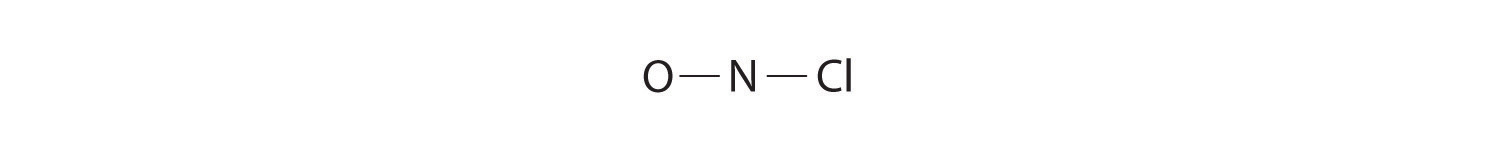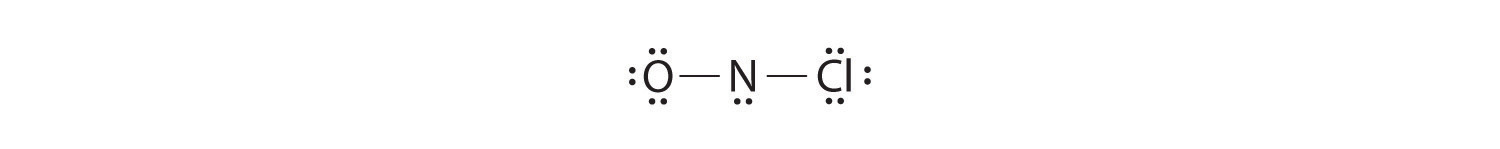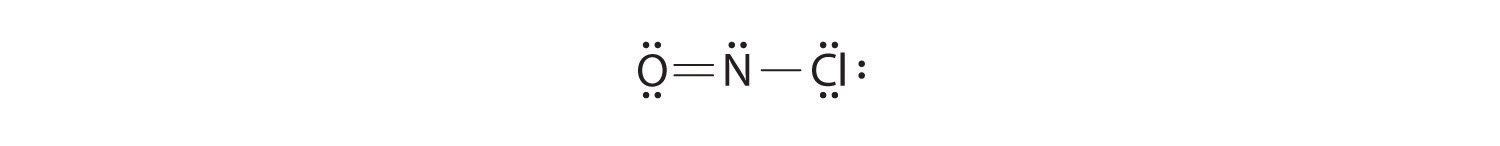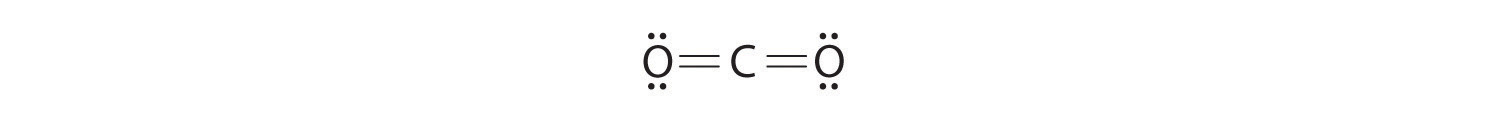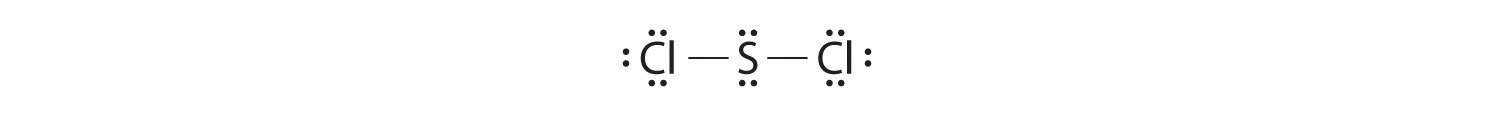8.2: Covalent Bonding and Lewis Structures
- Page ID
- 158456
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
The ideal gas law is easy to remember and apply in solving problems, as long as you get the proper values a
Before proceeding do a quick review of "Covalent Bonds and Molecules", see section 2.5 Chemical Bonds of this LibreText. Note in that section we covered different ways to represent molecules, like molecular formulas, structural formulas, and condensed formulas. In this section we are going to focus on structural formulas and develop a type of structural formula called the Lewis Dot Structure. In a Lewis Structure, you draw a molecular structure that accounts for the valence electrons of all the participating atoms, showing the connectivity (bonds) as lines between atoms, and lone electrons as dots. In section 8.6 you will use this Lewis dot structure to predict the three dimensional geometry of a molecule.
Lewis Dot Symbols for Atoms
In the last chapter we recognized that electrons reside in orbitals, and that the elements in the same groups have similar chemical properties because they have the same valence electron configurations, and therefore, the same number of valence electrons. For the representative elements (s and p blocks) we have the following valence shell electron configurations. The Lewis Electron Dot Symbol for atoms shows the number of valence electrons by representing the core electrons with the elements symbol and the valence electrons as dots as shown in Table \(\PageIndex{1}\)
|
Table \(\PageIndex{1}\) : valence electron configurations showing the number of valence electrons for the representative elements and the corresponding Lewis Electron Dot Symbol.
For the main group elements, the group number (using the "A" convention) reveals the number of valence electrons without having to write the full electron configuration. In addition, the s and p-block elements have 4 orbitals (one-s orbital and three p-orbitals), and so the convention is to put one electron in each orbital before pairing them, and to only allow two electrons in an orbital (Pauli Exclusion principle). This means that there are 8 electrons around the central atom if we are filling S & p orbitals, which is the foundation of the octet rule (see below).
For transition elements, valence electrons include the ns and (n-1)d electrons. However, elements in the same group still have similar chemical properties because their electron configurations are similar. For example, the electron configuration for vanadium is:
V: 1s22s23s23p64s23d2
Core electrons: 1s22s23s23p6 = [Ar]
Valence electrons: 4s23d2
Lewis Dot Structure Conventions
There are two types of electrons, lone electrons and bonding electrons. The lone electron is only attracted to one nuclei, the bonding electrons are shared between two nuclei.
- Lone electrons are represented by a dot
- Bonding electrons are represented by a line connecting two nuclei
- Lone Electrons
- Single Electron X\(\large\cdot\) (Single electron in orbit of nuclei X, note; this orbital could accept an additional electron)
- Pair of Electrons X: (spin coupled pair of electrons in an orbital of nuclei X)
- Bonding Electron Pairs
- Single bond X-Y (two electrons shared in a bonding orbital between nuclei X & Y)
- Double bond X=Y (four electrons shared in two bonding orbitals between nuclei X & Y)
- Triple bond X\(\equiv \)Y (6 electrons shared in three bonding orbitals between nuclei X & Y)
Lewis Dot Structures For Diatomics
Hydrogen
Since hydrogen has one valence electron and only s orbitals, each orbital in each hydrogen is more energetically stable if it had a full orbital. As each orbital would like two electrons, two hydrogen atoms come together and share their electrons, providing the single bonded diatomic hydrogen molecule. Note: Hydrogen only forms one bond in most compounds.

Halogens
Halogen are in group 7A and, therefore, have 7 valence electrons. Since they have both s and p orbitals, the most energetically stable atoms will have a full octet. If two halogens are within close proximity to each other, the best way to obtain that octet is if they both shared one electron with the other. For example,
X=F, Cl, Br, or I:
\[\underset{\Large{\cdot\cdot}}
{\overset{\Large{\cdot\cdot}}
{\textrm{:X}\cdot}} +\underset{\Large{\cdot\cdot}}
{\overset{\Large{\cdot\cdot}}
{\cdot\textrm{X:}}} \rightarrow \underset{\Large{\cdot\cdot}}
{\overset{\Large{\cdot\cdot}}
{\textrm{:X}}} -\underset{\Large{\cdot\cdot}}
{\overset{\Large{\cdot\cdot}}
{\textrm{X:}}} \]
Although there are exceptions that we will discuss later, we can say that halogen typically form 1 bond and have three lone pairs of electrons.
Oxygen
Since oxygen has six valence electrons each, it needs two more electrons to complete it's full octet. It is not adequate to share just one electron to form one bond with another oxygen, and so, two electrons are shared to form a double bond as shown below:
\[\underset{\Large{\cdot\cdot\,}}
{\overset{\Large{\cdot}}
{\textrm{:O}\cdot}}+\underset{\Large{\cdot\cdot\,}}
{\overset{\Large{\cdot}}
{\cdot\textrm{O:}}}\rightarrow \underset{\Large{\cdot\cdot\,}}
{
{\textrm{:O}}}=\underset{\Large{\cdot\cdot\,}}
{
{\textrm{O:}}}\]
Although this is not that always the case, we can see that oxygen and other elements in Group 6A will tend to form 2 bonds and have 2 lone pair of electrons.
Nitrogen
Nitrogen has five valence electrons and needs three more electrons to achieve a full octet. Therefore, nitrogen will form a triple bond with another nitrogen atom in which each is contributing three electrons to the bond.
\[\underset{\Large{\cdot\,}}
{\overset{\Large{\cdot}}
{\textrm{:N}\cdot}}+\underset{\Large{\cdot\,}}
{\overset{\Large{\cdot}}
{\cdot\textrm{N:}}}\rightarrow
{
{\textrm{:N}}}\equiv
{
{\textrm{N:}}}\]
Again, this may not always be the case, but it seems that nitrogen and other elements in group 5A will tend to form three bonds and have one lone pair. As you can see, the electron configuration and the number of valence electrons help us predict the number of covalent bonds formed for diatomic molecules. For more complex molecules involving more than one type of atom or for polyatomic ions that are held together by covalent bonds, it becomes slightly more complex to determine the Lewis Dot Structure. However, there is a systematic way to approach those problems as we will discuss here in the following section.
Lewis Dot Structures for Molecules and Atoms
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:
1. Arrange the atoms to show specific connections. When there is a central atom, it is usually the least electronegative element in the compound. Chemists usually list this central atom first in the chemical formula (as in CCl4 and CO32−, which both have C as the central atom), which is another clue to the compound’s structure. Hydrogen and the halogens are almost always connected to only one other atom, so they are usually terminal rather than central.
Note the Pattern
The central atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are usually terminal.
2. Determine the total number of valence electrons in the molecule or ion. Add together the valence electrons from each atom. (Recall from Chapter 2 that the number of valence electrons is indicated by the position of the element in the periodic table.) If the species is a polyatomic ion, remember to add or subtract the number of electrons necessary to give the total charge on the ion. For CO32−, for example, we add two electrons to the total because of the −2 charge.
3. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. In H2O, for example, there is a bonding pair of electrons between oxygen and each hydrogen.
4. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). These electrons will usually be lone pairs.
5. If any electrons are left over, place them on the central atom. We explain in Section 4.6 that some atoms are able to accommodate more than eight electrons.
6. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple (double or triple) bonds to the central atom to achieve an octet. This will not change the number of electrons on the terminal atoms.
Now let’s apply this procedure to some particular compounds, beginning with one we have already discussed.
Example \(\PageIndex{1}\)
Draw the Lewis Structure for H2O
Solution
1. Because H atoms are almost always terminal, the arrangement within the molecule must be HOH.
2. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons.
3. Placing one bonding pair of electrons between the O atom and each H atom gives H:O:H, with 4 electrons left over.
4. Each H atom has a full valence shell of 2 electrons.
5. Adding the remaining 4 electrons to the oxygen (as two lone pairs) gives the following structure:
This is the Lewis structure we drew earlier. Because it gives oxygen an octet and each hydrogen two electrons, we do not need to use step 6.
Example \(\PageIndex{2}\)
Draw the Lewis Structure for OCl-
Solution
1. With only two atoms in the molecule, there is no central atom.
2. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons.
3. Placing a bonding pair of electrons between O and Cl gives O:Cl, with 12 electrons left over.
4. If we place six electrons (as three lone pairs) on each atom, we obtain the following structure:
Each atom now has an octet of electrons, so steps 5 and 6 are not needed. The Lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. OCl− is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant.
Example \(\PageIndex{3}\)
Draw the Lewis Structure for H2CO
Solution
1. Because carbon is less electronegative than oxygen and hydrogen is normally terminal, C must be the central atom. One possible arrangement is as follows:
2. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons.
3. Placing a bonding pair of electrons between each pair of bonded atoms gives the following:
Six electrons are used, and 6 are left over.
4. Adding all 6 remaining electrons to oxygen (as three lone pairs) gives the following:
Although oxygen now has an octet and each hydrogen has 2 electrons, carbon has only 6 electrons.
5. There are no electrons left to place on the central atom.
6. To give carbon an octet of electrons, we use one of the lone pairs of electrons on oxygen to form a carbon–oxygen double bond:
Both the oxygen and the carbon now have an octet of electrons, so this is an acceptable Lewis electron structure. The O has two bonding pairs and two lone pairs, and C has four bonding pairs. This is the structure of formaldehyde, which is used in embalming fluid.
An alternative structure can be drawn with one H bonded to O. Formal charges, discussed later in this section, suggest that such a structure is less stable than that shown previously.
Write the Lewis electron structure for each species.
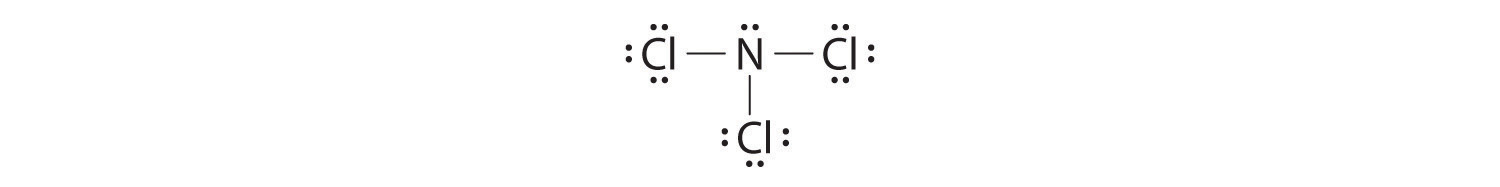
- NCl3
- S22−
- NOCl
Given: chemical species
Asked for: Lewis electron structures
Strategy:
Use the six-step procedure to write the Lewis electron structure for each species.
Solution:
-
Nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. The nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26 valence electrons. Using 2 electrons for each N–Cl bond and adding three lone pairs to each Cl account for (3 × 2) + (3 × 2 × 3) = 24 electrons. Rule 5 leads us to place the remaining 2 electrons on the central N:
Nitrogen trichloride is an unstable oily liquid once used to bleach flour; this use is now prohibited in the United States.
-
In a diatomic molecule or ion, we do not need to worry about a central atom. Each sulfur atom (group 16) contains 6 valence electrons, and we need to add 2 electrons for the −2 charge, giving a total of 14 valence electrons. Using 2 electrons for the S–S bond, we arrange the remaining 12 electrons as three lone pairs on each sulfur, giving each S atom an octet of electrons:
-
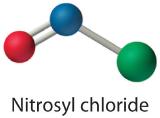
Because nitrogen is less electronegative than oxygen or chlorine, it is the central atom. The N atom (group 15) has 5 valence electrons, the O atom (group 16) has 6 valence electrons, and the Cl atom (group 17) has 7 valence electrons, giving a total of 18 valence electrons. Placing one bonding pair of electrons between each pair of bonded atoms uses 4 electrons and gives the following:
Adding three lone pairs each to oxygen and to chlorine uses 12 more electrons, leaving 2 electrons to place as a lone pair on nitrogen:
-
Because this Lewis structure has only 6 electrons around the central nitrogen, a lone pair of electrons on a terminal atom must be used to form a bonding pair. We could use a lone pair on either O or Cl. Because we have seen many structures in which O forms a double bond but none with a double bond to Cl, it is reasonable to select a lone pair from O to give the following:
All atoms now have octet configurations. This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, reddish-orange gas.
Exercise
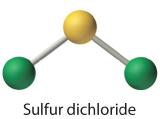
Write Lewis electron structures for CO2 and SCl2, a vile-smelling, unstable red liquid that is used in the manufacture of rubber.
Answer:
Contributors
- Original Article
- Modified by Ronia Kattoum (UA of Little Rock)
Lewis Dot Structures
The following handout should be downloaded and looked at before watching the videos.
Handout 1.1: Lewis Dot Structure Technique:
http://chemwiki.ucdavis.edu/@api/deki/files/60817/vsepr-1-Review.pdf
Handout 1.2: Lewis Dot Structure Worksheet:
http://chemwiki.ucdavis.edu/@api/deki/files/61154/vsepr1_WorkSheet.pdf
Lewis dot structure for Sulfur Trioxide (SO3). You should look at Handout 1 while watching.
Video 8.5.1: Please look at Handout 1: Lewis dot structure technique while watching this video. Note steps 1 and 2 are switched but all the rest are the same.
Lewis dot structure for Sulfite (SO3-2). You should look at Handout 1 while watching.
Video 8.5.2: Please look at Handout 1: Lewis dot structure technique while watching this video. Note steps 1 and 2 are switched but all the rest are the same.