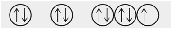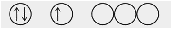7: The Structure of Atoms and Periodic Trends
- Page ID
- 169577
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Pauli Exclusion Principle
Exercise \(\PageIndex{1}\)
Which of the following statements concerning the Pauli exclusion principle is/are CORRECT?
- If two electrons occupy the same orbital they must have opposite spins.
- No two electrons in an atom can have the same four quantum numbers.
- Electrons with opposing spins are attracted to each other.
a. 1 only b. 2 only c. 3 only d. 1 and 2 e. 1,2, and 3
- Answer
-
d. 1 and 2
Quantum Numbers and Electron Configuration
Exercise \(\PageIndex{2}\)
How many electrons can be described by the following quantum numbers: n = 4, l = 2, = 2, ms = \(-1\frac{1}{2}\)?
a. 1 b. 2 c. 6 d. 10 e. 18
- Answer
-
a. 1
Exercise \(\PageIndex{3}\)
How many electrons can be described by the following quantum numbers: n = 3, l = 2, ml = 1?
a. 1 b. 2 c. 6 d. 10 e. 18
- Answer
-
b. 2
Exercise \(\PageIndex{4}\)
How many electrons can be described by the quantum numbers n= 3 and l= 2?
a. 14 b. 6 c. 2 d. 18 e. 10
- Answer
-
e. 10
Exercise \(\PageIndex{5}\)
What is the maximum number of electrons that can occupy the n = 3 shell?
a. 2 b. 8 c. 18 d. 32 e. 50
- Answer
-
a. 2
Exercise \(\PageIndex{6}\)
What is the maximum number of electrons that can occupy one s orbital?
a. 1 b. 2 c. 6 d. 10 e. 14
- Answer
-
b. 2
Exercise \(\PageIndex{7}\)
Which of the following orbital occupancy designations is incorrect?
a. 2s2 b. 3d6 c. 1s2 d. 4p3 e. 3d12
- Answer
-
e. 3d12
Exercise \(\PageIndex{8}\)
The maximum number of electrons that can be accommodated in a p subshell is
a. 10 b. 2 c. 14 d. 1 e. 6
- Answer
-
e. 6
Exercise \(\PageIndex{9}\)
Which of the following electron configurations is not allowed?
- 1s22s22p2
- 1s22s22p4
- 1s22s3
- 1s22s22p63s2
- 1s22s22p5
- Answer
-
c. 1s22s3
Exercise \(\PageIndex{10}\)
Which of the following sets of quantum numbers is allowed?
- n = 2, l = 1, = \(+\frac{1}{2}\), ms = \(-1\frac{1}{2}\)
- n = 3, l = 2, = +1, ms = +1
- n = 4, l = 1, = 0, ms = \(-1\frac{1}{2}\)
- n = 4, l = 3, = –1, ms = 0
- n = 5, l = 2, = +2, ms = –1
- Answer
-
c. n = 4, l = 1, = 0, ms = \(-1\frac{1}{2}\)
The Aufbau Process
Exercise \(\PageIndex{1}\)
The procedure by which electrons are assigned to (or built up into) orbitals is known as the ____ principle.
a. Aufbau b. Bohr c. Planck d. Hund e. Pauli
- Answer
-
a. Aufbau
Exercise \(\PageIndex{2}\)
Which of the following statements is true concerning the electron configuration [Xe]6p2?
- This configuration cannot be the ground-state electron configuration for a Ba atom because it violates the Pauli exclusion principle.
- This configuration cannot be the ground-state electron configuration for a Ba atom because it violates Hund's rule.
- This configuration is the ground-state electron configuration for a Ba atom.
- This configuration cannot be the ground-state electron configuration for a Ba atom because it violates the Heisenberg uncertainty principle.
- This configuration cannot be the ground-state electron configuration for a Ba atom because it violates the Aufbau principle.
- Answer
-
e. This configuration cannot be the ground-state electron configuration for a Ba atom because it violates the Aufbau principle.
Exercise \(\PageIndex{3}\)
According to the Aufbau principle, which of the following subshells is typically filled next after the 4s subshell?
a. 3d b. 4s c. 3p d. 2p e. 2s
- Answer
-
a. 3d
Exercise \(\PageIndex{4}\)
Which of the following statements is/are CORRECT for an oxygen atom?
- The effective nuclear charge felt by a 2s electron is greater than that felt by a 1s electron.
- The effective nuclear charges felt by 2s and 2p electrons are identical.
- The effective nuclear charge felt by a 2p electron is less than that felt by a 2s electron.
a. 1 only b. 2 only c. 3 only d. 1 and 3 e. 1, 2, and 3
- Answer
-
c. 3 only
Exercise \(\PageIndex{5}\)
The small, but important, energy differences between 3s, 3p, and 3d electrons is a consequence of
- the number of electrons they can hold
- their principal quantum number
- the Heisenberg uncertainty principle
- thier effective nuclear charge
- Hund's rule
- Answer
-
d. thier effective nuclear charge
Exercise \(\PageIndex{6}\)
Which of the following statements is true?
- Outer electrons efficiently shield one another from nuclear charge.
- Core electrons effectively shield outer electrons from nuclear charge.
- Valence electrons are the most difficult of all electrons to remove.
- Core electrons have the lowest ionization energies of all electrons.
- Valence electrons in the outermost shell of all elements have the highest ionization energy.
- Answer
-
b. Core electrons effectively shield outer electrons from nuclear charge.
Electron Configuration
Exercise \(\PageIndex{1}\)
Which of the following elements is found in the d-block of the periodic table?
- Ir
- Tb
- Li
- Cl
- None of these
- Answer
-
a Ir
Exercise \(\PageIndex{2}\)
An element that has the same ground state valence-shell electron configuration as thallium is
a. gallium b. carbon c. krypton d. cesium e. magnesium
- Answer
-
a. gallium
Exercise \(\PageIndex{3}\)
How many valence electrons does an arsenic atom have?
a. 5 b. 8 c. 7 d. 2 e. 33
- Answer
-
a. 5
Exercise \(\PageIndex{4}\)
How many unpaired electrons are found in the ground state electron configuration of Barium (Ba)?
a. 0 b. 1 c. 2 d. 3 e. 5
- Answer
-
a. 0
Exercise \(\PageIndex{5}\)
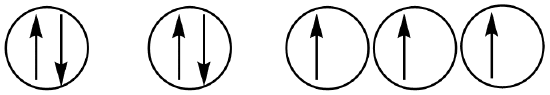
Which of the following orbital diagrams represents a paramagnetic atom?
1s 2s 2p
a. 1 only b. 2 only c. 3 only d. 1 and 2 e. 2 and 3
- Answer
-
e. 2 and 3
Exercise \(\PageIndex{6}\)
Which of the following atoms is diamagnetic in its ground state?
- Mercury (Hg)
- Tin (Sn)
- Rhenium (Re)
- Berkelium (Bk)
- Phosphorus (P)
- Answer
-
a. Mercury (Hg)
Exercise \(\PageIndex{7}\)
Which of the following orbital diagrams represents a diamagnetic atom?
1s 2s 2p
- Answer
-
a.
Exercise \(\PageIndex{8}\)
Which atom has the ground state electronic configuration 1s22s22p63s23p64s23d3?
a. Ga b. V c. As d. Nb e. none
- Answer
-
b. V
Exercise \(\PageIndex{9}\)
Which of the following elements has the ground state electron configuration [Ar]3d104s1?
a. Cu b. Zn c. Ge d. Ag e. Cd
- Answer
-
a. Cu
Exercise \(\PageIndex{10}\)
What is the ground-state electron configuration of sulfur (S)?
a. [Ne]3s33sp3 b. [Ar]3s23p4 c. [Ar]3p6 d. [Ne]3s23p4 e. [Ar]3p6
- Answer
-
d. [Ne]3s23p4
Exercise \(\PageIndex{11}\)
Which of the following electron configurations corresponds to the ground state of an atom of a transition element?
- 1s22s22p1
- 1s22s22p63s23p63d104s24p3
- 1s22s22p63s23p63d14s2
- 1s22s22p63s23p64s1
- 1s22s22p63s23p4
- Answer
-
c. 1s22s22p63s23p63d14s2
Exercise \(\PageIndex{12}\)
The complete electron configuration of tin is _____.
- 1s22s22p63s23p64s23d104p65s24d105p2
- 1s22s22p63s23p64s23d104p65s24d105p2
- 1s22s22p63s23p64s23d104d104p2
- 1s22s22p63s23p64s23d104p65s24d105d105p2
- None of these
- Answer
-
a. 1s22s22p63s23p64s23d104p65s24d105p2
Exercise \(\PageIndex{13}\)
Hund's rule states that the most stable arrangement of electrons (for a ground state electron configuration)
- has a filled valence shell of electrons.
- has three electrons per orbital, each with identical spins.
-
has values greater than or equal to +1.
-
has the maximum number of unpaired electrons, all with the same spin.
-
has two electrons per orbital, each with opposing spins.
- Answer
-
d. has the maximum number of unpaired electrons, all with the same spin.
Exercise \(\PageIndex{14}\)
All of the following ground-state electron configurations are correct except
- V: [Ar]4s24d3
- K: [Ar]4s1
- Sb: [Kr]4d105s25p3
- Cr: [Ar]3d54s1
- Te: [Kr]4d105s25p4
- Answer
-
a. V: [Ar]4s24d3
Exercise \(\PageIndex{15}\)
What noble gas core precedes the valence shell ground state electron configuration for potassium (K)?
a. [Ar] b. [Rn] c. [Kr] d. [Ne] e. [Xe]
- Answer
-
a. [Ar]
Exercise \(\PageIndex{16}\)
Which ground-state electron configuration is incorrect?
- Br: [Ar]3d104s24p5
- K: [Ar]4s1
- Ni: [Ar]3d5
- Mg: 1s22s22p63s2
- Co: [Ar]3d74s2
- Answer
-
c. Ni: [Ar]3d5
Exercise \(\PageIndex{17}\)
Which element has the following ground state electron configuration?
a. Be b. O c. Li d. Si e. N
- Answer
-
e. N
Exercise \(\PageIndex{18}\)
Which element has the following ground state electron configuration?
3d 4s
[Ar]
a. Sc b. Ni c. Co d. Fe e. V
- Answer
-
a. Sc
Exercise \(\PageIndex{19}\)
Which element has the following ground state electron configuration?
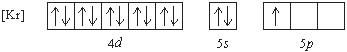
a. In b. Y c. Nb d. Tl e. Ga
- Answer
-
a. In
Exercise \(\PageIndex{20}\)
Which is the correct valence shell orbital box notation for the ground state electron configuration of Fe?
3d 4s
- Answer
-
a.

Exercise \(\PageIndex{21}\)
What is a possible set of quantum numbers for an unpaired electron in the orbital box diagram below?
- n = 1, l = 1, = –1, ms = \(+\frac{1}{2}\)
- n = 4, l = 2, = –1, ms = \(-1\frac{1}{2}\)
- n = 5, l = 2, = –2, ms = \(+\frac{1}{2}\)
- n = 5, l = 0, = 0, ms = \(-1\frac{1}{2}\)
- n = 5, l = 1, = –1, ms = \(+\frac{1}{2}\)
- Answer
-
e. n = 5, l = 1, = –1, ms = \(+\frac{1}{2}\)
Electron Configuration of Ions
Exercise \(\PageIndex{1}\)
For which of the following atoms is the 2+ ion diamagnetic in the ground state?
a. Ni b. Fe c. Zn d. Mn e. Cu
- Answer
-
c. Zn
Exercise \(\PageIndex{2}\)
Which of the following elements in its 1+ ionic state has the ground state electron configuration [Kr]4d10?
a. Ru b. Au c. Ag d. In e. Cd
- Answer
-
c. Ag
Exercise \(\PageIndex{3}\)
If the ground state electron configuration of an element is [Ar]3d104s24p5, what is the typical charge on the monatomic anion of the element?
a. 4+ b. 2+ c. 1- d. 2- e. 3-
- Answer
-
c. 1-
Exercise \(\PageIndex{4}\)
What is the ground state electron configuration for Cr3+?
- [Ar]
- [Ar]3d74s2
- [Ar]3d14s2
- [Ar]3d24s1
- [Ar]3d3
- Answer
-
e. [Ar]3d3
Exercise \(\PageIndex{5}\)
What is the ground state electron configuration for Sn2+?
- [Kr]4d105s2
- [Kr]4d105p2
- [Kr]5s2
- [Kr]4d105s25p2
- [Kr]4d105s25p4
- Answer
-
a. [Kr]4d105s2
Exercise \(\PageIndex{6}\)
Which of the given ions have the same ground state electron configuration: S2–, N3–, Mg2+, and Br–?
- N3– and Mg2+
- S2–, N3–, and Br–
- S2– and Br–
- Mg2+ and Br–
- S2–, N3–, Mg2+, and Br–
- Answer
-
a. N3– and Mg2+
Exercise \(\PageIndex{7}\)
Which of the following has the same (total) number of electrons as Ar?
a. Na+ b. Ca2+ c. Ga3+ d. O2- e. none
- Answer
-
b. Ca2+
Exercise \(\PageIndex{8}\)
What is the ground-state electron configuration of the chloride ion?
- 1s22s22p6
- 1s22s22p63s23p2
- 1s22s22p63s23p6
- 1s22s22p63s2
- 1s22s22p63s23p4
- Answer
-
Add texts here. Do not delete this text first.
Exercise \(\PageIndex{9}\)
What 2– ion has the following ground state electron configuration?
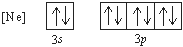
- Oxide ion
- Nitride ion
- Fluoride ion
- Sulfide Ion
- Magnesium Ion
- Answer
-
d. Sulfide Ion
Exercise \(\PageIndex{10}\)
What 2+ ion has the following ground state electron configuration?
a. Mn2+ b. Co2+ c. Ni2+ d. Cu2+ e. Ge2+
- Answer
-
b. Co2+
Exercise \(\PageIndex{11}\)
Which of the following ions has the given ground state electron configuration?
a. Cd2+ b. Sr2+ c. Zn2+ d.Sn2+ None of these
- Answer
-
d. Sn2+
Exercise \(\PageIndex{12}\)
The ground-state electron configuration of a Ni2+ ion is 1s22s22p63s23p63d8 . Therefore, Ni2+ is
- paramagnetic with two unpaired electrons.
- diamagnetic.
- paramagnetic with one unpaired electron.
- paramagnetic with four unpaired electrons.
- paramagnetic with five unpaired electrons.
- Answer
-
a. paramagnetic with two unpaired electrons.
Exercise \(\PageIndex{13}\)
Which of the following cations has the same number of unpaired electrons as Fe2+?
a. Ni2+ b. Fe3+ c. Cr2+ d. Mn2+ e. Co2+
- Answer
-
c. Cr2+
Atomic Radius
Exercise \(\PageIndex{1}\)
Which of the following statements is true of atomic radii?
- They decrease down a group and remain constant across a period.
- They decrease down a group and increase across a period.
- They increase down a group and increase across a period.
- They increase down a group and remain constant across a period.
- They increase down a group and decrease across a period.
- Answer
-
e. They increase down a group and decrease across a period.
Exercise \(\PageIndex{2}\)
An atom of which of the following elements has the smallest atomic radius?
a. F b. Rb c. Ca d. Ge e. P
- Answer
-
a. F
Exercise \(\PageIndex{3}\)
Which of the following atoms of elements has the largest atomic radius?
a. Ga b. In c. Al d. Tl e. B
- Answer
-
d. Tl
Exercise \(\PageIndex{4}\)
Rank the following atoms in order decreasing atomic radii: Br, Bi, Be, B.
- B > Br > Be > Bi
- Be > B > Br > Bi
- Bi > Be > Br > B
- Be > Br > Bi > B
- B > Bi > Be > Br
- Answer
-
a. B > Br > Be > Bi
Exercise \(\PageIndex{5}\)
Place the following atoms in order of increasing atomic radii: Se, Sb, Br, and Te.
- Br < Se < Te < Sb
- Se < Br < Sb < Te
- Se < Br < Te < As
- Sb < Te < Se < Br
- Te < Sb < Se < Br
- Answer
-
a. Br < Se < Te < Sb
Ionization Energy (Ionization Potential)
Exercise \(\PageIndex{6}\)
An atom of which of the following elements has the smallest ionization energy?
a. At b. Bi c. Pb d. Cs e. Po
- Answer
-
d. Cs
Exercise \(\PageIndex{7}\)
Which of the following statements is/are CORRECT?
- For any element, the second ionization energy is larger than the first ionization energy.
- Ionization energy is a positive value for all elements.
- Ionization energy increases down a group of the periodic table.
a. 1 only b. 2 only c. 3 only d. 1 and 2 e. 1, 2 and 3
- Answer
-
d. 1 and 2
Exercise \(\PageIndex{8}\)
The change in energy for which of the following processes corresponds to the first ionization energy of beryllium?
- Be(g) → Be+(g) + e–
- Be(s) → Be+(s) + e–
- Be(s) → Be+(g) + e–
- Be(g) → Be2+(g) + 2e–
- Be(s) + e– → Be–(s)
- Answer
-
a. Be(g) → Be+(g) + e–
Exercise \(\PageIndex{9}\)
Which of the following equations corresponds to the second ionization of magnesium?
- Mg(g) → Mg+(g) + e–
- Mg(g) → Mg+(g) + e–
- Mg(g) → Mg+(g) + e–
- Mg(g) → Mg2+(g) + 2e–
- Mg(g) + e– → Mg–(g)
- Answer
-
c. Mg(g) → Mg+(g) + e–
Exercise \(\PageIndex{10}\)
For which one of the following elements is the second ionization energy over ten times larger than its first ionization energy?
a. B b. N c. Li d. Ne e. Cu
- Answer
-
c. Li
Exercise \(\PageIndex{11}\)
Arrange F, Cl, and Br in order of their increasing first ionization energies.
- F < Cl < Br
- Cl < F < Br
- Cl < Br < F
- Br < F < Cl
- Br < Cl < F
- Answer
-
e. Br < Cl < F
Electron Affinity
Exercise \(\PageIndex{12}\)
The change in energy for the following reaction is referred to as the ____ for boron.
B(g) + e– → B–(g)
- oxidation number
- electron affinity
- electronegativity energy
- first ionization energy
- second ionization energy
- Answer
-
b. electron affinity
Exercise \(\PageIndex{13}\)
An atom of which of the following elements has the most negative electron affinity?
a. K b. Sb c. Cl d. Br e. O
- Answer
-
c. Cl
Exercise \(\PageIndex{14}\)
_____ have no affinity for electrons.
- Transition metals
- s-block elements
- Main group nonmetals
- Noble gases
- Semiconductors
- Answer
-
d. Noble gases
Exercise \(\PageIndex{15}\)
According to the general trend in electron affinities, which group (or family) of elements tends to form the most stable anions in the gas phase?
- Noble gases
- Halogens
- Transition metals
- Alkaline earth metals
- Alkali metals
- Answer
-
b. Halogens
Ionic Radius
Exercise \(\PageIndex{16}\)
Which group of the periodic table of elements forms only 2+ ions?
- group 1A
- group 2A
- group 1B
- group 7A
- group 8A
- Answer
-
b. group 2A
Exercise \(\PageIndex{17}\)
Rank the following ions in order of decreasing ionic radii: Be2+, Ca2+, Mg2+.
- Be2+, Ca2+, Mg2+
- Mg2+, Be2+, Ca2+
- Ca2+, Be2+, Mg2+
- Be2+, Mg2+, Ca2+
- Ca2+, Mg2+, Be2+
- Answer
-
e. Ca2+, Mg2+, Be2+
Exercise \(\PageIndex{18}\)
Place the following ions in order from smallest to largest ionic radii: K+, Na+, Mg2+, and Al3+.
- Al3+ < Mg2+ < Na+ < K+
- Na+ < Mg2+ < Al3+ < K+
- K+ < Mg2+ < Na+ < Al3+
- K+ < Al3+ < Mg2+ < Na+
- Mg2+ < Al3+ < Na+ < K+
- Answer
-
a. Al3+ < Mg2+ < Na+ < K+
Exercise \(\PageIndex{19}\)
Which of the following species has the largest radius?
a. Cl– b. P c. K– d. Br– e. Ca2+
- Answer
-
d. Br-
Trends
Exercise \(\PageIndex{1}\)
Which of the following elements would be expected to have chemical and physical properties most similar to Iodine (I)?
- Fluorine (F)
- Aluminum (Al)
- Magnesium (Mg)
- Rubidium (Rb)
- Krypton (Kr)
- Answer
-
a. Fluorine (F)
Exercise \(\PageIndex{2}\)
What is the charge formed by alkaline earth metals when they react with nonmetals?
a. +1 b. −1 c. +2 d. −2 e. +3
- Answer
-
c. +2
Exercise \(\PageIndex{3}\)
A metal oxide forms when potassium reacts with oxygen. What is the most likely formula of this metal oxide?
a. KO b. K2O c. K2O3 d. KO2 e. KO3
- Answer
-
a. KO
Exercise \(\PageIndex{4}\)
A metal phosphide forms when potassium reacts with elemental phosphorus. What is the most likely formula of this metal phosphide?
a. KP b. K3P c. K2P3 d. K3P2 e. KP3
- Answer
-
b. K3P
Exercise \(\PageIndex{5}\)
A metal halide forms when potassium reacts with elemental chlorine. What is the most likely formula of this metal halide?
a. KCl b. KCl2 c. K2Cl d. KCl3 e. K3Cl2
- Answer
-
b. KCl2