4.2: Limiting and Excess Reagents
- Page ID
- 166316
Limiting Reagent
Exercise \(\PageIndex{1}\)
The box below shows a group of nitrogen and hydrogen molecules that will react to produce ammonia, NH3. What is the limiting reagent?
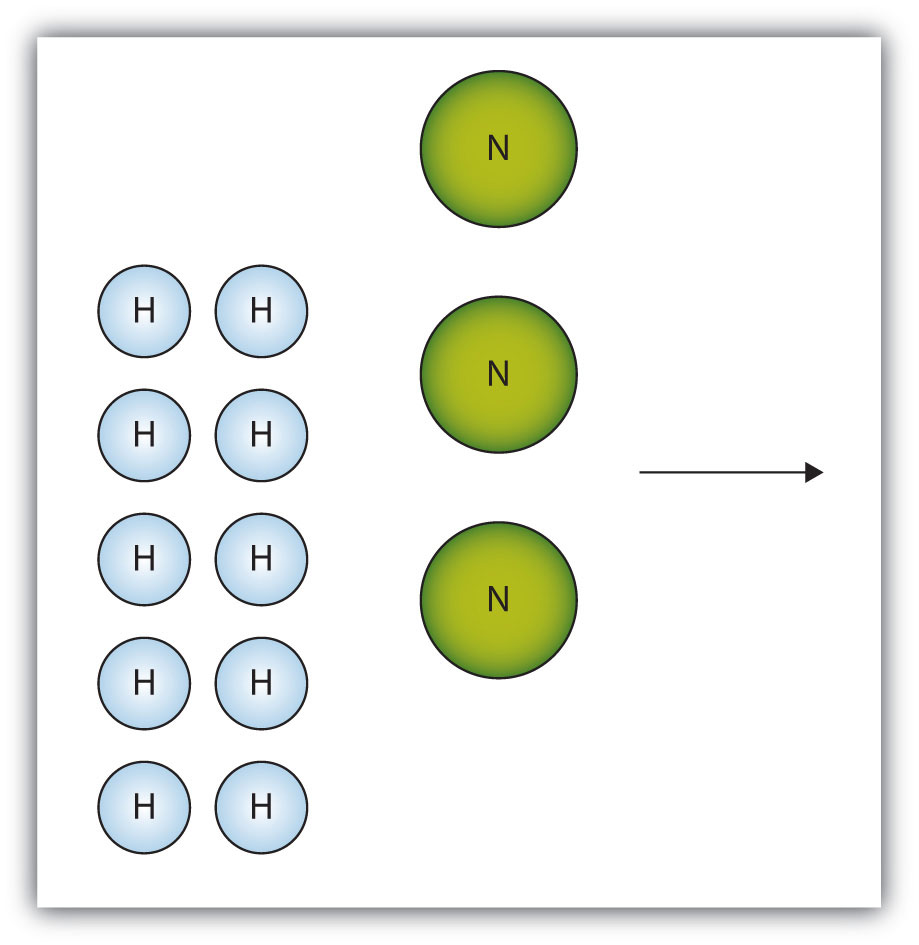
- Answer
-
Nitrogen is the limiting reagent.
Exercise \(\PageIndex{2}\)
Given the statement “20.0 g of methane is burned in excess oxygen,” is it obvious which reactant is the limiting reagent?
- Answer
-
Yes; methane is the limiting reagent.
Calculations with Limiting Reagent
Exercise \(\PageIndex{3}\)
Acetylene (C2H2) is formed by reacting 7.08 g of C and 4.92 g of H2.
2C(s) + H2(g) → C2H2(g)What is the limiting reagent? How much of the other reactant is in excess?
- Answer
-
C is the limiting reagent; 4.33 g of H2 are left over.
Exercise \(\PageIndex{4}\)
To form the precipitate PbCl2, 2.88 g of NaCl and 7.21 g of Pb(NO3)2 are mixed in solution. How much precipitate is formed? How much of which reactant is in excess?
- Answer
-
6.06 g of PbCl2 are formed; 0.33 g of NaCl is left over.

