4: Stoichiometry: Quantitative Information about Chemical Reactions
- Page ID
- 166236
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Moles and Grams from Grams
Exercise \(\PageIndex{1}\)
For the balanced chemical equation
Al(s) + 3Ag+(aq) → Al3+(aq) + 3Ag(s)how many moles and grams of Ag are produced when 0.661 mol of Al are reacted?
- Answer
-
\(0.661 mol Al*\left ( \frac{3 mol Ag}{1 mol Al} \right )= 1.98 mol Ag\)
\(0.661 mol Al*\left ( \frac{3 mol Ag}{1 mol Al} \right )*\left ( \frac{107.8682 g Ag}{1 mol Ag} \right )=214 grams Ag\)
Exercise \(\PageIndex{2}\)
For the balanced chemical reaction
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(ℓ)how many moles and grams of H2O are produced when 0.669 mol of NH3 react?
- Answer
-
1.00 mol H2O, 18.0 grams of H2O
Moles and grams from grams
Exercise \(\PageIndex{3}\)
For the balanced chemical reaction
4NaOH(aq) + 2S(s) + 3O2(g) → 2Na2SO4(aq) + 2H2O(ℓ)how many moles and grams of Na2SO4 are formed when 1.22 grams of O2 react?
- Answer
-
\(1.22 g O_{2}*\left ( \frac{1 mol O_{2}}{31.988 g O_{2}} \right )*\left ( \frac{2 mol Na_{2}SO_{4}}{3 mol O_{2}} \right )= 0.0254 mol Na_{2}SO_{4}\)
\(1.22 g O_{2}*\left ( \frac{1 mol O_{2}}{31.988 g O_{2}} \right )*\left ( \frac{2 mol Na_{2}SO_{4}}{3 mol O_{2}} \right )*\left ( \frac{142.04314 g Na_{2}SO_{4}}{1 mol Na_{2}SO_{4}} \right )=3.61 g Na_{2}SO_{4}\)
Exercise \(\PageIndex{4}\)
For the balanced chemical reaction
4KO2(s) + 2CO2(g) → 2K2CO3(s) + 3O2(g)determine the number of moles and grams of both products formed when 6.88 grams of KO2 react.
- Answer
-
Moles: 0.0484 mol K2CO3, 0.0726 mol O2
Grams: 6.69 grams K2CO3, 2.32 grams O2
Limiting Reagent
Exercise \(\PageIndex{1}\)
The box below shows a group of nitrogen and hydrogen molecules that will react to produce ammonia, NH3. What is the limiting reagent?
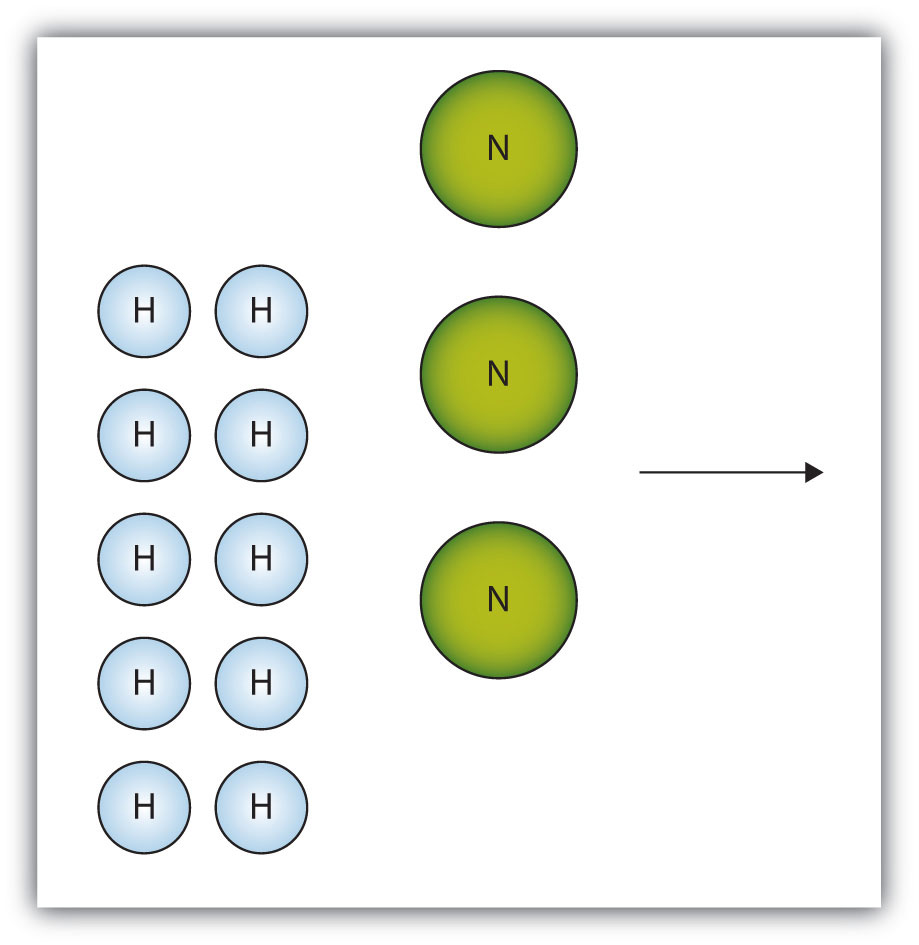
- Answer
-
Nitrogen is the limiting reagent.
Exercise \(\PageIndex{2}\)
Given the statement “20.0 g of methane is burned in excess oxygen,” is it obvious which reactant is the limiting reagent?
- Answer
-
Yes; methane is the limiting reagent.
Calculations with Limiting Reagent
Exercise \(\PageIndex{3}\)
Acetylene (C2H2) is formed by reacting 7.08 g of C and 4.92 g of H2.
2C(s) + H2(g) → C2H2(g)What is the limiting reagent? How much of the other reactant is in excess?
- Answer
-
C is the limiting reagent; 4.33 g of H2 are left over.
Exercise \(\PageIndex{4}\)
To form the precipitate PbCl2, 2.88 g of NaCl and 7.21 g of Pb(NO3)2 are mixed in solution. How much precipitate is formed? How much of which reactant is in excess?
- Answer
-
6.06 g of PbCl2 are formed; 0.33 g of NaCl is left over.
Vocabulary
Exercise \(\PageIndex{1}\)
- What is the difference between the theoretical yield and the actual yield?
-
What is the difference between the actual yield and the percent yield?
- Answer
-
- Theoretical yield is what you expect stoichiometrically from a chemical reaction; actual yield is what you actually get from a chemical reaction.
- Actual yield is what you actually get from a chemical reaction; percent yield is the percent ratio of actual yield to the theoretical yield, meaning it is a ratio that determines the accuracy of the actual yield from an experiment.
Percent, Actual, and Theoretical Yield
Exercise \(\PageIndex{2}\)
A worker isolates 2.675 g of SiF4 after reacting 2.339 g of SiO2 with HF. What are the theoretical yield and the actual yield? What is the percent yield?
SiO2(s) + 4HF(g) → SiF4(g) + 2H2O(ℓ)
- Answer
-
theoretical yield = 4.052 g; actual yield = 2.675 g
percent yield = 66.02%
Exercise \(\PageIndex{3}\)
A chemist decomposes 1.006 g of NaHCO3 and obtains 0.0334 g of Na2CO3. What are the theoretical yield and the actual yield? What is the percent yield?
2NaHCO3(s) → Na2CO3(s) + H2O(ℓ) + CO2(g)
- Answer
-
theoretical yield = 0.635 g; actual yield = 0.0334 g
percent yield = 5.26%
Combustion Analysis
Exercise \(\PageIndex{1}\)
The principal component of mothballs is naphthalene, a compound with a molecular mass of about 130 amu, containing only carbon and hydrogen. A 3.000-mg sample of naphthalene burns to give 10.3 mg of CO2. Determine its empirical and molecular formulas.
- Answer
-
- First find mass of Carbon and Hydrogen
\(m_{c}=\left ( \frac{1 g}{1000 mg} \right )\left ( \frac{1 mol CO_{2}}{44.01 g CO_{2}} \right )\left ( \frac{1 mol C}{1 mol CO_{2}} \right )\left ( \frac{12.01 g C}{1 molC} \right )\left ( \frac{1000 mg}{1 g} \right )=2.811 mg C\)
\(m_{H}=\left ( 3.000 mg - 2.811 mg \right )=0.189 mg H\)
- Second find the moles of Carbon and Hydrogen.
\(n_{1}=\frac{2.811*10^{-3}g}{12.01g*mol^{-1}}=2.34*10^{-4}mol)
\(n_{2}=\frac{0.189*10^{-3}g}{1.008g*mol^{-1}}=1.875*10^{-4}mol\)
- Now solve for Empirical Formula
\(C = \frac{2.34*10^{-4}mol}{1.875*10^{-4}mol}=1.2492\)
\(H = \frac{1.875*10^{-4}mol}{1.875*10^{-4}mol}=1\)
Multiply each by 4 to get the smallest integer. (1.2492 * 4 = 4.9968 or 5). C5H4
- Lastly solve for the molecular formula using empirical formula
\(M_{1}=5(M_{C})+4(M_{H})\)
\(M_{1}=5(12.01 g*mol^{-1})+4(1.008 g*mol^{-1})=64.085 amu\)
\(n_{f}=\frac{130 amu}{64.085 amu}=2\)
Now multiply the empirical formula by 2 to get the molecular formula. C10H8.
Exercise \(\PageIndex{2}\)
Mesitylene is a liquid hydrocarbon. Burning 0.115 g of the compound in oxygen gives 0.379 g of CO2 and 0.1035 g of H2O. What is the empirical formula of mesitylene?
- Answer
-
C3H4
Exercise \(\PageIndex{3}\)
A 0.025-g sample of a compound composed of boron and hydrogen, with a molecular mass of ~28 amu, burns spontaneously when exposed to air, producing 0.063 g of B2O3. What are the empirical and molecular formulas of the compound.
- Answer
-
Empirical Formula: BH3
Molecular Formula: B2H6
Molarity
Exercise \(\PageIndex{1}\)
What is the molarity of an NaI solution that contains 4.5 g of NaI in 21.0 mL of solution?
- 1.4 M
- 0.030 M
- 0.0047 M
- 0.00014 M
- 0.21 M
- Answer
-
a. 1.4 M
\(n_{NaI}=4.5 g NaI (\frac{1 mol}{149.89424})=0.03 mol NaI\)
\(M = \frac{mol}{L}=\frac{0.03}{21*10^{-3}L}=1.429 M\)
Exercise \(\PageIndex{2}\)
What volume of 0.742 M Na2CO3 solution contains 44.9 g of Na2CO3?
- 0.314 L
- 6.41 × 103 L
- 0.571 L
- 3.53 × 103 L
- 1.75 L
- Answer
-
c. 0.571 L
Exercise \(\PageIndex{3}\)
How many moles of sulfate ions are there in a 0.650-L solution of 0.312 M Al2(SO4)3?
- 0.203 mol
- 0.608 mol
- 6.25 mol
- 0.0676 mol
- 1.44 mol
- Answer
-
b. 0.608 mol
Exercise \(\PageIndex{4}\)
What mass of Na2CO3 is present in 0.700 L of a 0.396 M Na2CO3 solution?
- 29.4 g
- 74.2 g
- 42.0 g
- 187 g
- 60.0 g
- Answer
-
a. 29.4 g
Dilutions
Exercise \(\PageIndex{5}\)
In order to dilute 77.1 mL of 0.778 M HCl to 0.100 M, the volume of water that must be added is
- 67.2 mL
- 9.91 mL
- 6 * 102 mL
- 1.01 * 10-3 mL
- 5.23 * 102 mL
- Answer
-
e. 5.23 * 102 mL
\(M_{1}*V_{1}=M_{2}*V_{2}\)
\(V_{2}=\frac{\left (M_{1}*V_{1} \right )}{M_{2}}=\frac{\left ( 0.778 M * \left ( 77.1 * 10^{-3}L \right )\right )}{0.100 M}= .5998 L\)
Exercise \(\PageIndex{6}\)
A dilute solution is prepared by transferring 35.00 mL of a 0.6363 M stock solution to a 900.0 mL volumetric flask and diluting to mark. What is the molarity of this dilute solution?
- 0.02474 M
- 0.04949 M
- 0.1636 M
- 0.006186 M
- 0.3182 M
- Answer
-
a. 0.02474 M
Exercise \(\PageIndex{7}\)
What volume of 1.27 M HCl is required to prepare 197.4 mL of 0.456 M HCl?
- 5.5 * 102 mL
- 3.41 * 102 mL
- 70.9 mL
- 0.0141 mL
- 1.14 * 102 mL
- Answer
-
c. 70.9 mL
Finding pH
Exercise \(\PageIndex{1}\)
What is the pH of 4.1 × 10−3 M HCl(aq)? Is this solution acidic or basic?
- Answer
-
\(pH = -log(H^{+})=-log(4.1*10^{-3})=2.39\)
Acidic Solution
Exercise \(\PageIndex{2}\)
What is the pH of a solution that has a H+ concentration of 2.4 * 10-9 M. Is this solution acidic or basic?
- Answer
-
pH = 8.62
Basic Solution
Finding Hydronium Ion Concentration
Exercise \(\PageIndex{3}\)
The pH of a vinegar solution is 4.15. What is the H3O+ concentration of the solution?
- Answer
-
Note Hydronium ion, H30+, and H+ are all the same thing.
\(H^{+}=10^{-pH}=10^{-4.15}=7.08 * 10^{-5}M\)
Exercise \(\PageIndex{4}\)
What is the H+ concentration of a solution with a pH of 12.05.
- Answer
-
8.91 * 10-13 M
Stoichiometry in Solutions
Exercise \(\PageIndex{1}\)
Add exercises text here.
- Answer
-
Add texts here. Do not delete this text first.
Exercise \(\PageIndex{1}\)
The concentration of sulfate in a sample of wastewater is to be determined by using gravimetric analysis. To a 100.0-mL sample of the wastewater is added an excess of barium nitrate, forming the insoluble barium sulfate (233.4 g/mol) according to the balanced equation given below. The solid barium sulfate is dried, and its mass is measured to be 0.9951 g. What was the concentration of sulfate in the original wastewater sample?
SO42–(aq) + Ba(NO3)2(aq) → BaSO4(s) + 2NO3–(aq)
- Answer
-
\(n_{BaSO_{4}}=\frac{0.9951g BaSO_{4}}{233.4 g/mol}=4.265 * 10^{-3} M\)
Exercise \(\PageIndex{2}\)
Zn reacts with hydrochloric acid.
Zn(s) + 2 HCl(aq) → ZnCl2(aq) + H2(g)
What volume of 3.05 M HCl(aq) will react with 25.0 g Zn(s)?
- Answer
-
0.251 L
Exercise \(\PageIndex{3}\)
What minimum mass of cobalt (II) nitrate must be added to 60.0 mL of a 0.0999 M phosphate solution in order to completely precipitate all of the phosphate as solid cobalt (II) phosphate?
2PO43–(aq) + 3Co(NO3)2(aq) → Co3(PO4)2(s) + 6NO3–(aq)
- Answer
-
1.64 g
Titrations
Exercise \(\PageIndex{4}\)
The reaction of HCl with NaOH is represented by the equation
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
What volume of 0.614 M HCl is required to titrate 18.8 mL of 0.619 M NaOH?
- Answer
-
19.0 mL
\(V_{HCl}=V_{NaOH}\left ( \frac{M_{NaOH}}{M_{HCl}} \right )=18.8*\left ( \frac{0.619 M}{0.614 M} \right )=18.95 mL\)
Exercise \(\PageIndex{5}\)
What volume of 0.955 M HCl, in milliliters, is required to titrate 2.152 g of Na2CO3 to the equivalence point?
Na2CO3(aq) + 2 HCl(aq) → H2O(ℓ) + CO2(g) + 2 NaCl(aq)
- Answer
-
42.5 mL HCl
\(moles Na_{2}CO_{3}=\frac{2.152g}{105.98844g/mol}=0.0203 moles Na_{2}CO_{3}\)
\[moles HCl = 2*0.0203=0.0406 moles HCl\]
\[V=\frac{0.0406 moles HCl}{0.955 M}=0.0425 L= 42.5 mL\]
Absorbance and Beer-Lambert Law
Exercise \(\PageIndex{1}\)
Which of the following is the correct representation for absorbance of a sample?
- A = -log T
- A = log (-T)
- A = 10T
- A = 10-T
- A = 10-1/T
- Answer
-
a. A = -log T
Exercise \(\PageIndex{2}\)
Calibration of a spectrophotometer using a series of green dye containing solutions provides a set of data which follows the Beer-Lambert Law, with a trendline of y = 1.398 × 103x (y-axis = absorbance, x-axis = molar concentration). What is the molar concentration of a solution with an absorbance of 0.8427?
- Answer
-
\[y = 1.398*10^{3}x\]
\[x = \frac{y}{1.398*10^{3}}\]
\[x = \frac{0.8427}{1.398*10^{3}}=6.028*10^{-4}mol/mL=0.6028M\]

