3.3: Isotopes
- Page ID
- 278750
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Atomic Number and Mass Number
When you study the periodic table, the first thing that you may notice is the number that lies above the symbol. This number is known as the atomic number, which identifies the number of protons in the nucleus of ALL atoms in a given element.
The symbol for the atomic number is designated with the letter Z. For example, the atomic number (z) for sodium (Na) is 11. That means that all sodium atoms have 11 protons. If you change the atomic number to 12, you are no longer dealing with sodium atoms, but magnesium atoms. Hence, the atomic number defines the element in question.
Recall that the nuclei of most atoms contain neutrons as well as protons. Unlike protons, the number of neutrons is not absolutely fixed for most elements. Atoms that have the same number of protons, and hence the same atomic number, but different numbers of neutrons are called isotopes. All isotopes of an element have the same number of protons and electrons, which means they exhibit the same chemical behavior. Because different isotopes of the same element haves different number of neutrons, each of these isotopes will have a different mass number(A), which is the sum of the number of protons and the number of neutrons in the nucleus of an atom.
Mass Number(A) = Number of Protons + Number of Neutrons
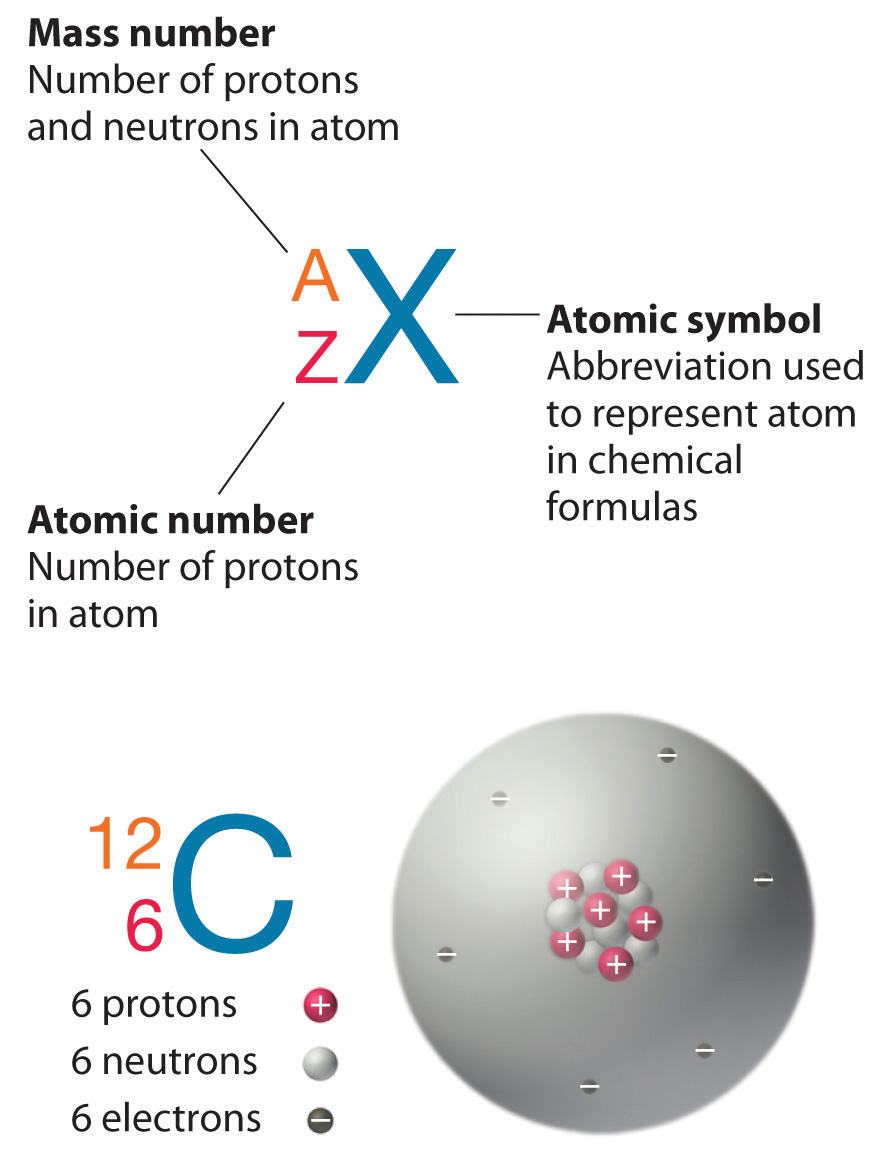
The element carbon (C) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. In a typical sample of carbon-containing material, 98.89% of the carbon atoms also contain 6 neutrons, so each has a mass number of 12. An isotope of any element can be uniquely represented as AZX, where X is the atomic symbol of the element, A is the mass number and Z is the atomic number. The isotope of carbon that has 6 neutrons is therefore 126C. The subscript indicating the atomic number is actually redundant because the atomic symbol already uniquely specifies Z. Consequently, it is more often written as 12C, which is written as “carbon-12,” (14C, would thus be written as “carbon-14.”) Nevertheless, the value of Z is commonly included in the notation for nuclear reactions because these reactions involve changes in Z.
Note
The value of the atomic number Z is often ommited as the symbol defines it, so 126C. is often written as 12C because any atom of the element carbon must have 6 protons, or it would not be carbon.
Atomic Mass Unit (Dalton)
The atomic mass unit (u, amu or Da) is essentially 1/12th the mass of a 12C atom (that was the definition before 2019, and it is now based on the 7 immutable constants). This is a very small number and can be related to the macroscopic world through the following conversion factor.
\[ 1 amu = 1.66054x10^{-24}g = 1.66054 \; \text{yg}\]
(yg=yoctogram, see section 2.7- SI prefixes)
The amu is also called the Dalton and large molecules like proteins, nucleic acid and large polymers are often expressed in Kilo Daltons (1kDA=103 Da) or Mega Daltons (1MDa=106 Da).
Molar Mass
The atomic mass unit is very small, as it is effectively a scale based on the mass of an atom, and in the world we live and breath in measurements are of the order of magnitude of a grams and kilograms, and so taking the reciprocal of 3.31 we can get a feel for how many atomic mass units are in a gram.
\[ 1g =\frac{amu}{1.6605x10^{-24}} = 6.0221x10^{23}amu \]
The value of 6.022x1023 is also known as Avogadro's constant, as representing the number of entities in the SI base unit of the mole (section 2.4.3)
\[1 \text{mole of anything} = 6.022x10^{23} \text{things}\]
The Molar Mass of a substance is the mass of one mole of that substance (6.02x1023 particles) and so the atomic weight in AMUs is to an atom what the molar mass is in grams to a mole of atoms. That is, one atom of 1H weighs 1 amu, while one mole of 1H weights 1g.
\[1 \text{atom H} = 1 amu \\ 1 \text{mole H} = 1 g\]
Isotopes
Isotopes are different atoms of the same element that contain different numbers of neutrons in their nucleus, and therefore they have different atomic masses. For a neutral atom the number of electrons = the number of protons.
\[_{26}^{54}\textrm{Fe} \text{ which is often written as } ^{54}\textrm{Fe} \\ \text{ has} \\ \text{ 26 protons, 28 neutrons and 22 electrons}\]
For a charged atom the number of protons is not equal to the number of electrons and you have an ion. There are two types of ions, cations [+] charged and anions [-] charged. We write the charge as a post superscript. The charge of an ion is the number of protons minus the number of electrons, as each proton has a charge of [+1] and each electron has a charge of [-1].
Cations
Number of electrons is less than the number of protons
\[_{26}^{54}\textrm{Fe}^{+2} \text{ which is often written as }^{54}\textrm{Fe}^{+2} \\ \text{ has} \\ \text{ 26 protons, 28 neutrons and 24 electrons}\]
Anions
Number of electrons is greater than the number of protons.
\[_{17}^{37}\textrm{Cl}^{-} \text{ which is often written as }^{37}\textrm{Cl}^- \\ \text{ has} \\ \text{ 17 protons, 20 neutrons and 18 electrons}\]
Example 2.2.1
An element with three stable isotopes has 82 protons. The separate isotopes contain 124, 125, and 126 neutrons. Identify the element and write symbols for the isotopes.
Given: number of protons and neutrons
Asked for: element and atomic symbol
Strategy:
- Refer to the periodic table and use the number of protons to identify the element.
- Calculate the mass number of each isotope by adding together the numbers of protons and neutrons.
- Give the symbol of each isotope with the mass number as the superscript and the number of protons as the subscript, both written to the left of the symbol of the element.
Solution:
A The element with 82 protons (atomic number of 82) is lead: Pb.
B For the first isotope, A = 82 protons + 124 neutrons = 206. Similarly, A = 82 + 125 = 207 and A = 82 + 126 = 208 for the second and third isotopes, respectively. The symbols for these isotopes are 20682Pb, 20782Pb, and 20882Pb, which also can also be symbolized as Pb-206, Pb-207, and Pb-208.
Exercise \(\PageIndex{1}\)
What is the mass number of a phosphorous atom with 16 neutrons
- Answer
-
31
Exercise \(\PageIndex{2}\)
How many protons, neutrons, and electrons are in As-74 atom?
- Answer
-
33 protons, 41 neutrons, 33 electrons
Exercise \(\PageIndex{3}\)
The actual mass of As-74 is 73.924 amu's. (1 amu= 1.66054 x 10-24 g)
A) What is the mass of As-74 in grams?
B) What is the mass of the arsenic atom relative to the carbon-12 atom?
- Answer
-
A) Mass of As-74 = (73.924 amu) x (1.66054 x 10-24 g/amu) = 1.2275 x 10-22 g
B) Mass of As-74/Mass of C-12 = 73.924 amu/12.000 amu= 6.1603 ... so, the mass of an As-74 atom is 6.1603 times heavier than a C-12 atom.
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Anonymous
- Modified by Joshua Halpern, Scott Sinex and Scott Johnson
- November Palmer
- Elena Lisitsyna (H5P interactive modules)

