8.6: An Explanation of Substituent Effects
- Page ID
- 469419
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Substituted rings are divided into two groups based on the type of the substituent that the ring carries:
- Activated rings: the substituents on the ring are groups that donate electrons.
- Deactivated rings: the substituents on the ring are groups that withdraw electrons.
Introduction
Examples of activating groups in the relative order from the most activating group to the least activating:
-NH2, -NR2 > -OH, -OR> -NHCOR> -CH3 and other alkyl groups
with R as alkyl groups (CnH2n+1)
Examples of deactivating groups in the relative order from the most deactivating to the least deactivating:
-NO2, -CF3> -COR, -CN, -CO2R, -SO3H > Halogens
with R as alkyl groups (CnH2n+1)
The order of reactivity among Halogens from the more reactive (least deactivating substituent) to the least reactive (most deactivating substituent) halogen is:
F> Cl > Br > I
The order of reactivity of the benzene rings toward the electrophilic substitution when it is substituted with a halogen groups, follows the order of electronegativity. The ring that is substituted with the most electronegative halogen is the most reactive ring (less deactivating substituent) and the ring that is substituted with the least electronegative halogen is the least reactive ring (more deactivating substituent), when we compare rings with halogen substituents. Also the size of the halogen effects the reactivity of the benzene ring that the halogen is attached to. As the size of the halogen increase, the reactivity of the ring decreases.
The direction of the reaction
The activating group directs the reaction to the ortho or para position, which means the electrophile substitutes for the hydrogen that is on carbon 2 or carbon 4. The deactivating group directs the reaction to the meta position, which means the electrophile substitutes for the hydrogen that is on carbon 3 with the exception of the halogens which are deactivating groups but direct the ortho or para substitution.
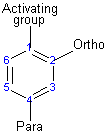
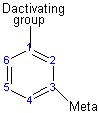
Substituents determine the reaction direction by resonance or inductive effect
Resonance effect is the conjugation between the ring and the substituent, which means the delocalization of the \(\pi\) electrons between the ring and the substituent. Inductive effect is the withdraw of the sigma ( the single bond ) electrons away from the ring toward the substituent, due to the higher electronegativity of the substituent compared to the carbon of the ring.
Activating groups (ortho or para directors)
When substituents such as -OH have an unshared pair of electrons, the resonance effect is stronger than the inductive effect which make these substituents stronger activators, since this resonance effect direct the electron toward the ring. In cases where the substituents is esters or amides, they are less activating because they form resonance structure that pull the electron density away from the ring.
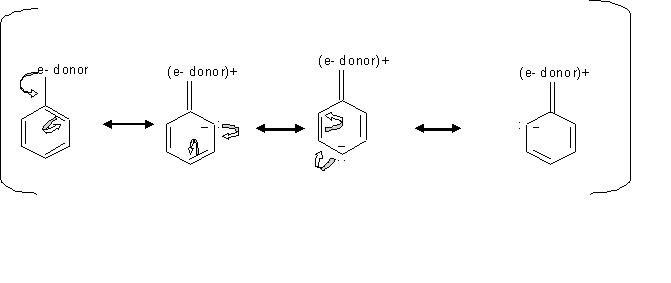
By looking at the mechanism above, we can see how electron donating groups direct electrophilic substitution to the ortho and para positions. Since the extra electron density is localized on the ortho and para carbons, these carbons are more likely to react with the electrophile.
Inductive effects of alkyl groups activate the direction of the ortho or para substitution, which is when s electrons gets pushed toward the ring.
Deactivating group (meta directors)
The deactivating groups deactivate the ring by the inductive effect in the presence of an electronegative atom that withdraws electron density away from the ring.
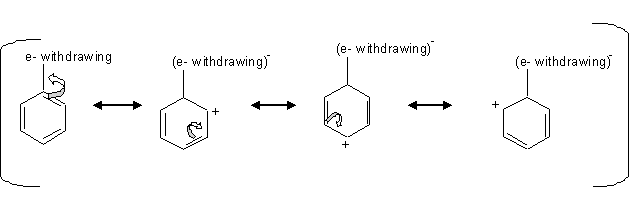
The mechanism above shows that when electron density is withdrawn from the ring, that leaves the carbons at the ortho, para positions with a parital positive charge which is unfavorable for the electrophile, so the electrophile attacks the carbon at the meta positions.
Halogens are an exception of the deactivating group that directs to the ortho or para substitution. The halogens deactivate the ring by inductive effect not by the resonance even though they have an unpaired pair of electrons. The unpaired pair of electrons gets donated to the ring, but the inductive effect pulls away the s electrons from the ring by the electronegativity of the halogens.
Substituents determine the reactivity of rings
The reaction of a substituted ring with an activating group is faster than the same reaction wtih benzene. On the other hand, a substituted ring with a deactivated group reacts slower than benzene.
Activating groups speed up reaction with electrophiles due to increased electron density on the ring. This stabilizes the intermediate carbocation, which decreases the activation energy for the reaction. On the other hand, deactivating groups withdraw electron density away from the carbocation formed in the intermediate step, increasing the activation energy, which slows down the reaction.
The CH3 Group is an ortho, para director
Alkyl groups are inductively donating, therefore are activators. This resulsts in o/p attack to form a tertiary arenium carbocation which speeds up the reaction.
The O-CH3 Group is an ortho, para director
The methoxy group is an example of groups that are ortho, para directors by having and oxygen or nitrogen adjacent to the aromatic ring. This same activation is present with alcohols, amines, esters and amides (with the oxygen or nitrogen attached to the ring, not the carbonyl).
Groups with an oxygen or nitrogen attached to the aromatic ring are ortho and para directors since the O or N can push electrons into the ring, making the ortho and para positions more reactive and stabilizing the arenium ion that forms. This causes the ortho and para products to form faster than meta. Generally, the para product is preferred because of steric effects.
Acyl groups are meta directors
Ketones are an example of groups that deactivate an aromatic ring through resonance. Similar deactivation also occurs with ammonium ions, nitro groups, aldehydes, nitriles, sulfonic acids, and groups with a carbonyl attached to the ring (amides, esters, carboxylic acids, and anhydrides).
Acyl groups are resonance deactivators. Ortho and para attack produces a resonance structure which places the arenium cation next to an additional cation. This destabilizes the arenium cation and slows down ortho and para reaction. By default the meta product forms faster because it lacks this destabilizing resonance structure.
Halogens
Halogens are an interesting hybrid case. They are ortho, para directors, but deactivators. Overall, they remove electron density from the ring, making it less reactive. However, due to their resonance donation to the ring, if it does react, it reacts primarily at ortho and para positions.

