8.4: Alkylation and Acylation of Aromatic Rings - The Friedel-Crafts Reaction
- Page ID
- 469417
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
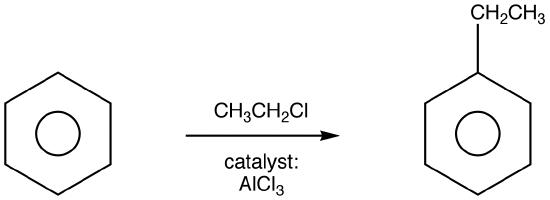
Friedel-Crafts Alkylation
Friedel-Crafts Alkylation was first discovered by French scientist Charles Friedel and his partner, American scientist James Crafts, in 1877. This reaction allowed for the formation of alkyl benzenes from alkyl halides, but was plagued with unwanted supplemental activity that reduced its effectiveness.
The mechanism takes place as follows:
Step 1:
.png?revision=1)
Step one creates a carbocation that acts as the electrophile in the reaction. This steps activates the haloalkane. Secondary and tertiary halides only form the free carbocation in this step.
Steps 2 and 3:
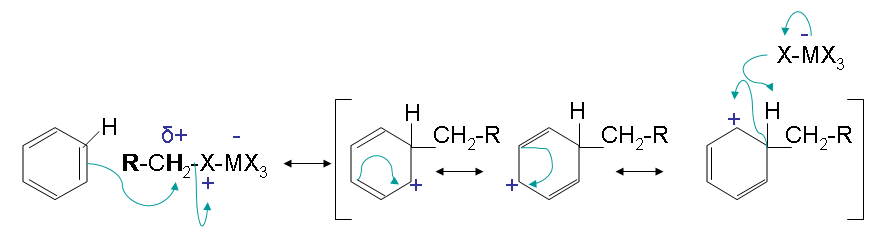
Step 2 has an electron pair from the aromatic ring attack the carbocation forming a new C-C bond. The arenium ion intermediate results with stabilization from multiple resonance forms. The loss of a proton then gives the neutral alkylated substitution product.
Final Products
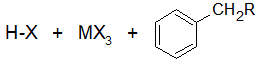
The reactivity of haloalkanes increases as you move up the periodic table and increase polarity. This means that an RF haloalkane is most reactive followed by RCl then RBr and finally RI. This means that the Lewis acids used as catalysts in Friedel-Crafts Alkylation reactions tend have similar halogen combinations such as BF3, SbCl5, AlCl3, SbCl5, and AlBr3, all of which are commonly used in these reactions.
Some limitations of Friedel-Crafts Alkylation
There are possibilities of carbocation rearrangements when you are trying to add a carbon chain greater than two carbons. The rearrangements occur due to hydride shifts and methyl shifts. For example, the product of a Friedel-Crafts Alkylation will show an iso rearrangement when adding a three carbon chain as a substituent. One way to resolve these problems is through Friedel-Crafts Acylation.
Also, the reaction will only work if the ring you are adding a substituent to is not deactivated. Friedel-Crafts fails when used with compounds such as nitrobenzene and other strong deactivating systems.
Friedel-Crafts reactions cannot be preformed then the aromatic ring contains a NH2, NHR, or NR2 substituent. The lone pair electrons on the amines react with the Lewis acid AlCl3. This places a positive charge next to the benzene ring, which is so strongly activating that the Friedel-Crafts reaction cannot occur.
Lastly, Friedel-Crafts alkylation can undergo polyalkylation. The reaction adds an electron donating alkyl group, which activates the benzene ring to further alkylation.
This problem does not occur during Friedel-Crafts Acylation because an acyl group is deactivating, thus prevents further acylations.
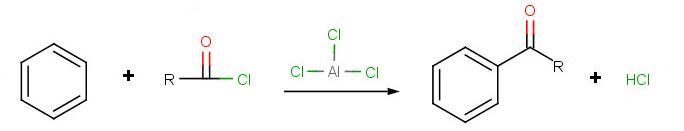
Friedel-Crafts Acylation
The goal of the reaction is the following:
.jpg?revision=1&size=bestfit&width=564&height=108)
The very first step involves the formation of the acylium ion which will later react with benzene:
The second step involves the attack of the acylium ion on benzene as a new electrophile to form one complex:
.jpg?revision=1&size=bestfit&width=385&height=116)
The third step involves the departure of the proton to reform aromaticity:
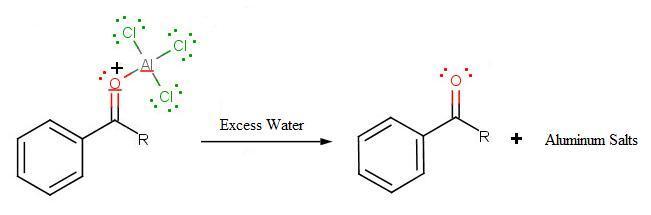
During the third step, AlCl4 returns to remove a proton from the benzene ring, which enables the ring to return to aromaticity. In doing so, the original AlCl3 is regenerated for use again, along with HCl. Most importantly, we have the first part of the final product of the reaction, which is a ketone. Thie first part of the product is the complex with aluminum chloride as shown:
.jpg?revision=1&size=bestfit&width=431&height=164)
The final step involves the addition of water to liberate the final product as the acylbenzene:
.jpg?revision=1&size=bestfit&width=521&height=172)
Because the acylium ion (as was shown in step one) is stabilized by resonance, no rearrangement occurs (unlike in Friedel-Crafts Alkylation reactions - see Limitation 1 above). Also, because of of the deactivation of the product, it is no longer susceptible to electrophilic attack and hence, no longer goes into further reactions (Limitation 3 above from Friedel-Crafts Alkylation reactions). However, as not all is perfect, Limitation 2 still prevails where Friedel-Crafts Acylation fails with strong deactivating rings.


.jpg?revision=1&size=bestfit&width=720&height=114)
.jpg?revision=1&size=bestfit&width=550&height=116)