7.8: Aromatic Heterocycles - Pyridine and Pyrrole
- Page ID
- 469412
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Aromatic Heterocycles
Many cyclic compounds have an element other than carbon present in the ring. Cyclic compounds that include one or more elements other than carbon are called a heterocycle. The most common heterocyclic compounds contain carbon along with nitrogen, oxygen, or sulfur. Because some heterocyclic compounds are aromatic, it is important to discuss how the inclusion of non-carbon atoms affects the determination of aromaticity.
Pyridine & Pyrimidine
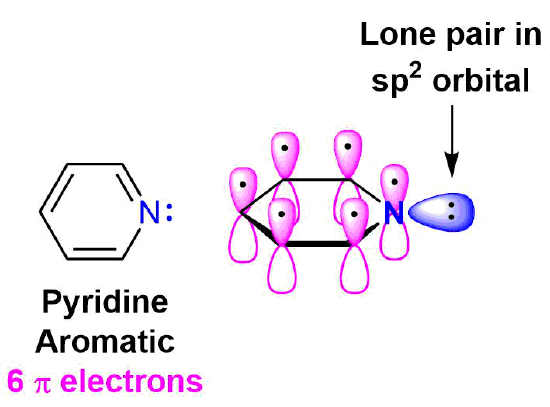
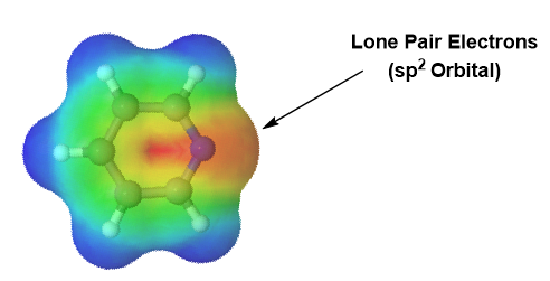
Pyridine is an example of a six-membered aromatic heterocycle and has an electronic structure similar to benzene. In the bonding picture of pyridine the five carbons and single nitrogen are all sp2 hybridized. All six of these atoms have a p orbital perpendicular to the plane of the ring and each contains one pi electron which allows the ring to be fully conjugated. This makes the pi electron count for pyridine 6 pi electrons. In much the same fashion as benzene, the 6 pi electrons follow the 4n + 2 rule and completely fill the bonding molecular orbitals which fulfills the electronic requirement for aromaticity. The lone pair electrons on pyridine's nitrogen are contained in a sp2 orbital that lies in the same plane as the ring and does not overlap with the p orbitals of the ring. The lone pair electrons are not part of the aromatic system and do not affect the p electron count. Pyridine's electrostatic potential map shows that the lone pair electrons, shown in a yellow/red color, are locked onto the nitrogen and not distributed around the ring.
Pyrimidine is an another six-membered aromatic heterocycle that is analogous to pyridine. Pyrimidine has four carbons atoms and two nitrogen atoms that are sp2 hybridized. Each of these atoms contributes a p orbital and a pi election allowing pyrimidine to be fully conjugated and aromatic. Both of the nitrogens in pyrimidine have lone pair electrons contained in a sp2 orbitals and are not involved in the aromatic system. An electrostatic potential map of pyrimidine shows that neither set of lone pair electrons is distributed around the ring.
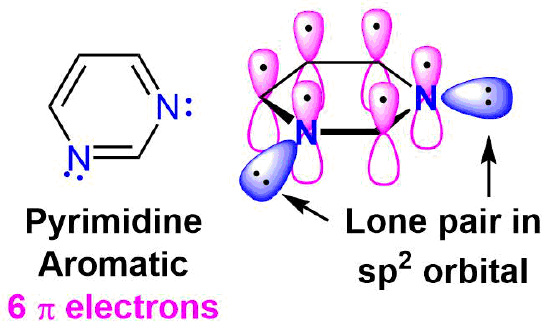
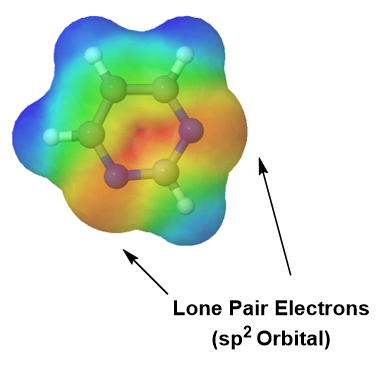
Pyrrole
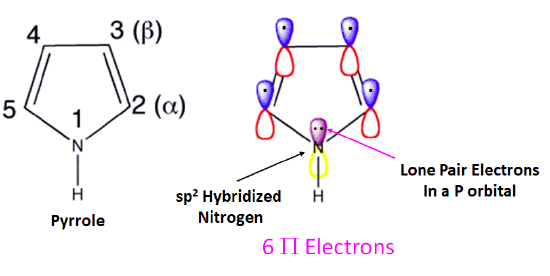
Pyrrole is a five-membered heterocyclic ring which has 5 p orbitals and six pi electrons contributing to its aromaticity. Each carbon in pyrrole contributes one p orbital and pi electron. The nitrogen in pyrrole contributes two pi electrons by becoming sp2 hybridized and placing its lone pair electrons into a p orbital. The electrostatic potential map of pyrrole show the nitrogen's lone pair electrons are distributed in the ring. Because the nitrogen lone pair is part of the aromatic sextet the electrons are very stable and are much less available for bonding to a proton (and if they do pick up a proton, the molecule loses aromaticity). Pyrrole is a very weak base: the conjugate acid, the pyrrolium ion, is a strong acid with a pKa of 0.4.
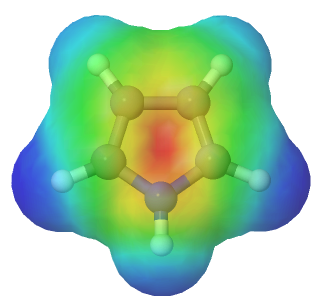
Imidazole
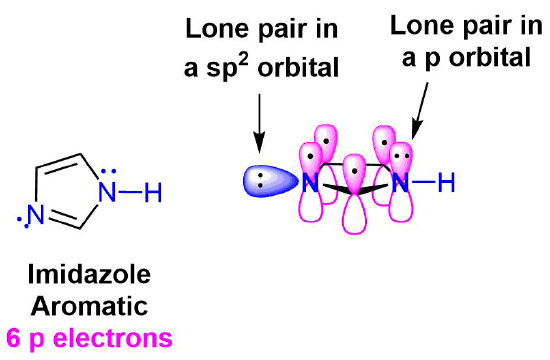
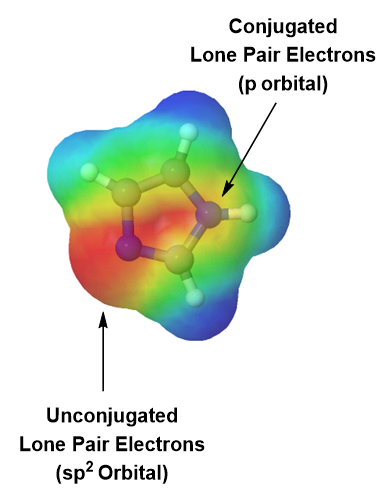
Imidazole is another five-membered heterocyclic ring which has 6 pi electrons and is aromatic. Both of the nitrogen atoms are sp2 hybridized. One nitrogen is pyridine-like because it is part of a double bond and adds one pi electron to the aromatic pi ring. The other nitrogen is not part of a double bond making it pyrrole-like allowing it to contribute 2 pi electrons from it lone pair electrons. When looking at the electrostatic potential map of imidazole, the lone pair electrons on the pyrrole-like nitrogen are distributed around the ring while the lone pair electrons on the pyridine-like nitrogen are non-conjugated and locked into place.
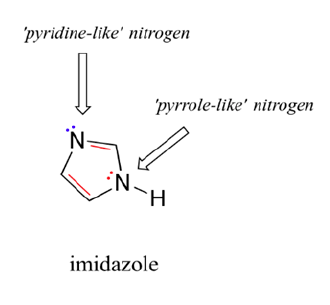
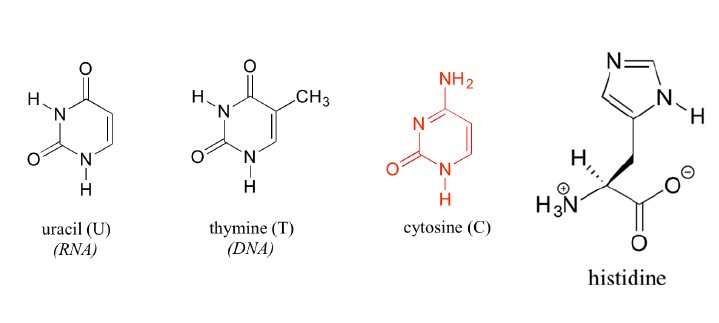
Pyrimidine and imidazole rings are particularly important in biological chemistry. Pyrimidine, for instance, is the parent ring system in cytosine, thymine, and uracil, three of the five heterocyclic amine bases found in nucleic acids. An aromatic imidazole ring is present in histidine, one of the
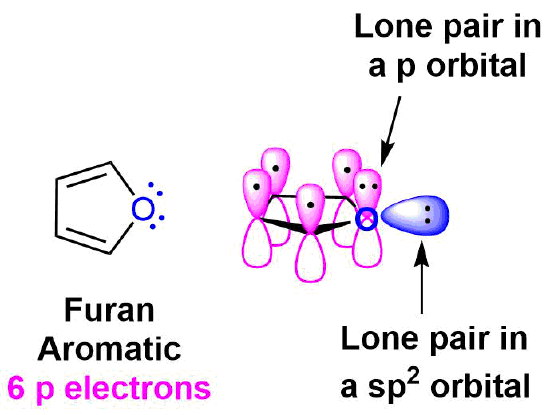
Determine if furan is aromatic. Draw an orbital diagram to support your answer.

- Answer
- For a molecule to be aromatic it must: Be cyclic, be planar, be fully conjugated, and have 4n+2 π electrons.
- Furan is obviously cyclic and can be assumed to be planar due to the constrains of having a five membered ring with two double bonds. To be fully conjugated each atom in the ring must have a p orbital. Although the oxygen has 4 electron groups and appears to be sp3 hybridized, it will become sp2 hybridized to gain the stability of conjugation. Becoming sp2 hybridized allows the oxygen atom to put a set of lone pair electrons into a p orbital allowing it to be part of the cyclic conjugation. These two pi electrons along with the 4 pi electrons provided by the two double bonds gives furan 6 pi electrons total. This follows the 4n + 2 rule making furan aromatic.