5.4: Sequence Rules for Specifying Configuration
- Last updated
- Save as PDF
- Page ID
- 451141
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Key Terms
Make certain that you can define, and use in context, the key terms below.
- absolute configuration
- R configuration
- S configuration
Study Notes
When designating a structure as R or S, you must ensure that the atom or group with the lowest priority is pointing away from you, the observer. The easiest way to show this is to use the wedge-and-broken-line representation. You can then immediately determine whether you are observing an R configuration or an S configuration.
To name the enantiomers of a compound unambiguously, their names must include the "handedness" of the molecule. The method for this is formally known as R/S nomenclature.
Introduction
The method of unambiguously assigning the handedness of molecules was originated by three chemists: R.S. Cahn, C. Ingold, and V. Prelog and, as such, is also often called the Cahn-Ingold-Prelog rules. In addition to the Cahn-Ingold system, there are two ways of experimentally determining the absolute configuration of an enantiomer:
- X-ray diffraction analysis. Note that there is no correlation between the sign of rotation and the structure of a particular enantiomer.
- Chemical correlation with a molecule whose structure has already been determined via X-ray diffraction.
However, for non-laboratory purposes, it is beneficial to focus on the R/S system. The sign of optical rotation, although different for the two enantiomers of a chiral molecule,at the same temperature, cannot be used to establish the absolute configuration of an enantiomer; this is because the sign of optical rotation for a particular enantiomer may change when the temperature changes.
Stereocenters are labeled R or S
The "right hand" and "left hand" nomenclature is used to name the enantiomers of a chiral compound. The stereocenters are labeled as R or S.
The Cahn-Ingold-Prelog rules of assign priorities the groups directly bonded to the chiral carbon. Having ranked the four groups attached to a chiral carbon, we describe the stereochemical configuration around the carbon by orienting the molecule so that the group with the lowest ranking (4) is given a dash bond to indicate it points directly away from us. We then look at the three remaining substituents, which now appear to radiate toward us which is shown by using wedge bonds. If a curved arrow drawn from the highest to second-highest to third-highest ranked substituent
Sequence Rules to Assign Priorities to Substituents
Before applying the R and S nomenclature to a stereocenter, the substituents must be prioritized according to the following rules:
Rule 1
First, examine at the atoms directly attached to the stereocenter of the compound. A atom with a higher atomic number takes precedence over a atom with a lower atomic number. Hydrogen is the lowest possible priority atom, because it has the lowest atomic number.
- The atom with higher atomic number has higher priority (I > Br > Cl > S > P > F > O > N > C > H).
- When comparing isotopes, the atom with the higher mass number has higher priority [18O > 16O or 15N > 14N or 13C > 12C or T (3H) > D (2H) > H].
Rule 2
If there are two or more substituents which have the same element directly attached to chiral carbon, proceed along the substituent chains until a point of difference is found. Determine which of the chains has the first connection to an atom with the highest priority (the highest atomic number). That chain has the higher priority.
For example: an ethyl substituent takes priority over a methyl substituent. At the connectivity of the stereocenter, both have a carbon atom, which are equal in rank. Going down the chains, a methyl has only has hydrogen atoms attached to it, whereas the ethyl has two hydrogen atoms and a carbon atom. The carbon atom on the ethyl is the first point of difference and has a higher atomic number than hydrogen; therefore the ethyl takes priority over the methyl.
Example \(\PageIndex{1}\)
Worked Exercise \(\PageIndex{1}\)
For the following pairs of substituents, determine which would have the higher and lower priority based on the Cahn-Ingold-Prelog rules. Explain your answer.
- Answer
-
A 1-methylethyl substituent takes precedence over an ethyl substituent. Connected to the first carbon atom, ethyl only has one other carbon, whereas the 1-methylethyl has two carbon atoms attached to the first; this is the first point of difference. Therefore, 1-methylethyl ranks higher in priority than ethyl, as shown below:
However:
Caution!!
Keep in mind that priority is determined by the first point of difference along the two similar substituent chains. After the first point of difference, the rest of the chain is irrelevant. - When looking for the first point of difference on similar substituent chains, one may encounter branching. If there is branching, choose the branch that is higher in priority. If the two substituents have similar branches, rank the elements within the branches until a point of difference.
-
Rule 3
For assigning priority, multiple bonds are treated as if each bond of the multiple bond is bonded to a unique atom. For example, an alkene substituent (CH2=CH-) has higher priority than an ethyl substituent (CH3CH2-). The alkene carbon priority is "two" bonds to carbon atoms and one bond to a hydrogen atom compared with the ethyl carbon that has only one bond to a carbon atom and two bonds to two hydrogen atoms. Similarly, alkyne substituent (HCC-) would have an even higher priority because the alkyne carbon is treated as if it is bonded to three carbons. This method remains the same with compounds containing a carbonyl (C=O) group. The carbon of an aldehyde substituent (O=CH-) is treated as if it is bonded to a hydrogen and two oxygen atoms.
Determining R or S Configuration Using a Molecular Model
In order to demonstrate how to determine the R/S configuration of the chiral carbon in the following molecule using molecular models, first construct a model of the bromoethanol structure:
First make a molecular model of a tetrahedral carbon with four different substituents. In many cases, this will appear as a carbon with four bonds with a different colored ball attached to each bond.
For the molecule in question, determine the location of the chiral carbon and assign CIP priorities to the substituents. In this case, Br gets the highest priority because it has the highest atomic number. The O in the OH substituent gets priority 2 and the C in CH3 gets priority 3. Lastly, H gets the lowest priority, 4, because it has the smallest atomic number.
Now take your molecular model and orientate it to match the molecule in question. Remember in the dash/wedge representation, two regular bonds are in the plane of the page. The wedge bond is coming toward you and the dashed bond is going away from you. If you were to hold a piece of paper directly in front of you, the substituents with the regular bond should both be touching the piece of paper. The dashed bond should be pointing behind the piece of paper and the wedge bond should be pointing in front.
In this structure, the bromine is going away from you, the hydrogen is coming toward you and the
hydroxide and methyl groups are in the plane of the page.
Then based on the position, assign each substituent on the chiral carbon a colored ball on your molecular model. In this case, bromine is going away so it is assigned the green ball. The hydrogen is coming toward you so it is assigned the blue ball. The last two substituents are in the plane of the page, however, the CH3 is positioned higher so it is assigned the red ball which leaves OH being assigned the black ball.
Lastly, grab onto the ball for the lowest priority substitutent, in this case the blue one, and point the other three substituents towards you. The three bonds should be angled towards you as if they all have wedge bonds. Assign the original substituents and their corresponding CIP priorities to the three colored balls. The green ball was assigned to Bromine which was given priority one. The OH was assigned to the black ball and given prioirty two. The CH3 was assigned to the red ball and given priority three. In this case the priorities are going counter clockwise so the chiral carbon has an S configuration.
Determining R or S Configuration Without a Molecular Model
If a molecular model cannot be used there are a couple of simple methods which can be applied if the dash/wedge bond system is being used.
After assigning CIP priorities, if the lowest priority substituent (4) is on the dash bond the configuration of substituents 1-3 can be assigned directly. As shown in the figure below, the configuration of substituents 1-3 does not change when moving to sight down the bond of substituent 4. In both cases, substituents 1-3 are ordered in a counterclockwise fashion which gives the chiral carbon an S configuration.
The opposite is true if the lowest priority substituent (4) is on the wedge bond. As shown in the figure below, the configuration of substituents 1-3 is inverted when moving to sight down the bond of substituent 4. When the lowest priority substituent is on the wedge bond, the configuration of substituents 1-3 can be assigned directly only if the direction is inverted. i.e. clockwise = S and counterclockwise = R.
With the lowest priority group in front, drawing an arc from 1 to 2 to 3 gives the reverse of the configuration.
However, if the lowest priority substituent is on one of the regular bonds when the dash/wedge system is being used then configurations are best assigned by changing perspectives. This method can also be used if the three-demensional configuration of the chiral carbon is represented. First, locate the chiral carbon and assign CIP priorities to its substituents. Then while perceiving the drawn molecule as a three-dimensional image, mentally change your perspective such that you are looking down the bond between the chiral carbon and the lowest CIP ranked substituent (#4). If done correctly, the bonds for substituents 1-3 should be coming towards you as wedge bonds. You can then follow the direction of the CIP priority numbers to determine the R/S configuration of the chiral carbon.
Locate the chiral carbon and assign CIP priorities to its substituents.
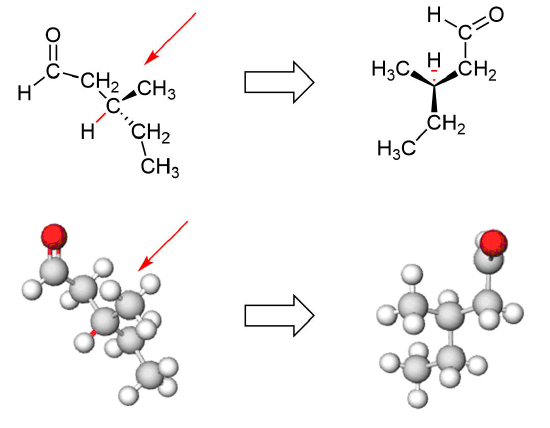
Mentally sight down the bond between the chiral carbon and the lowest CIP ranked substituent.
This bond is shown in Red.
Follow the direction of the CIP priority numbers to determine the R/S configuration.
Drawing the Structure of a Chiral Molecule from its Name
Draw the structure of (S)-2-Bromobutane:
1) Draw the basic structure of the molecule and determine the location of the chiral carbon.
2) Determine the chiral carbon's substituents and assign them a CIP priority.
-H (Priority 4)
-CH3 (Priority 3)
-CH2CH3 (Priority 2)
-Br (Priority 1)
3) Draw the chiral carbon in a dash/wedge form and add the lowest priority substituent to the wedge bond. In this case, the lowest priority substituent is -H.
4) Add the remaining substituents in a clockwise fashion for R and a counterclockwise fashion for S.
The molecule posed in this question has an S configuration so the remaining substituents are added in a counterclockwise fashion.
Exercise \(\PageIndex{1}\)
1) Orient the following so that the least priority (4) atom is paced behind, then assign stereochemistry (R or S).
2) Draw (R)-2-bromobutan-2-ol.
3) Assign R/S to the following molecule.
4) Which in the following pairs would have a higher CIP priority?
a) -H or -Cl
b) -Br or -I
c) -CH2OH or -OCH3
d) -CH2CH3 or -CH=CH2
e) -NH2 or -OH
5) Rank the following substituents in order of their CIP priority:
a) -H, -OCH3, -CH2OH, -OH
b) -OH, -CO2H, -CH=O, -CH2OH
c) -CN, -NH2, -CH=O, -NHCH3
d) -SH, -SCH3, -OH, -OOCH3
6) Determine if the chiral carbon in the following molecules have an R or S configuration. Red = Oxygen & Blue = Nitrogen.
a)
b)
- Answer
-
1) A is S and B is R.
2)
3) The stereocenter is R.
4)
a) -Cl
b) -I
c) -OCH3
d) -CH=CH2
e) -OH
5) Rank the following substituents in order of their CIP priority:
a) -OCH3, -OH, -CH2OH, -H,
b) -OH, -CO2H, -CH=O, -CH2OH
c) -NHCH3, -NH2, -CH=O, -CN
d) -SCH3, -SH, -OOCH3, -OH
6)
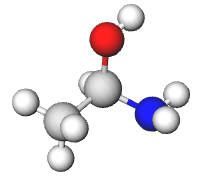
a) The chiral carbon is R. The four substituents of the chiral carbon are -OH (1), -NH2 (2), -CH3 (3), and -H (4). Then looking down the lowest priority bond, you should roughly see what appears in the picture below. The substituents with priorities 1-3 are ordered in a clockwise fashion so the chiral carbon is R.
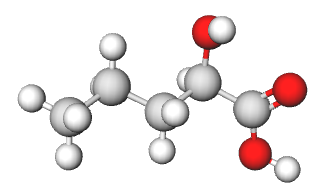
b) The chiral carbon is S. The four substituents of the chiral carbon are -CO2H (1), -OH (2), -CH2CH2CH3 (3), and -H (4). Then looking down the lowest priority bond, you should roughly see what appears in the picture below. The substituents with priorities 1-3 are ordered in a counterclockwise fashion so the chiral carbon is S.
Exercise \(\PageIndex{2}\)
Identify which substituent in the following sets has a higher ranking.
a) -H or -CH3
b) -CH2CH2CH3 or CH2CH3
c) -CH2Cl or CH2OH
- Answer
-
a) -CH3
b) -CH2CH2CH3
c) -CH2Cl
Exercise \(\PageIndex{3}\)
Identify which substituent in the following sets has a higher ranking.
a) -NH2 or -N=NH
b) -CH2CH2OH or -CH2OH
c) -CH=CH2 or -CH2CH3
- Answer
-
a) -N=NH
b) -CH2OH
c) -CH=CH2
Exercise \(\PageIndex{4}\)
Place the following sets of substituents in each group in order of lowest priority (1st) to highest priority (4th)
a) -NH2, -F, -Br, -CH3
b) -SH, -NH2, -F, -H
- Answer
-
a) -CH3 < -NH2 < -F, < -Br
b) -H < -NH2 < -F, < -SH
Exercise \(\PageIndex{5}\)
Place the following sets of substituents in each group in order of lowest priority (1st) to highest priority (4th)
a) -CH2CH3, -CN, -CH2CH2OH, -CH2CH2CH2OH
b) -CH2NH2, -CH2SH, -C(CH3)3, -CN
- Answer
-
a) -CH2CH3 < -CH2CH2OH < -CH2CH2CH2OH, < -CN
b) -C(CH3)3 < -CH2NH2 < -CN < -CH2SH
Exercise \(\PageIndex{6}\)
Assign the following chiral centers as R or S.
- Answer
-
a) S: I > Br > F > H. The lowest priority substituent is going backwards so following the highest priority, it goes left (counterclockwise).
b) R: Br > Cl > CH3 > H. Using a model kit, you need to rotate the H to the back position where the Br is. This causes the priority to go to the left (clockwise) when looking at it with the H in the back position. Alternatively, if you do not have a model kit, you can imagine the structure 3-dimensionally and since the lowest priority (H) is facing up (as drawn), if you look at it from below, starting with Br (1st priority) and moving towards Cl (2nd priority), you are moving right (clockwise) which represents R stereochemistry.
c) Neither R or S: Since there are two identical substituents (H’s) the molecule is achiral and cannot be assigned R or S.
Exercise \(\PageIndex{7}\)
Assign the following chiral centers as R or S.
- Answer
-
a) R: OH > CN (C triple bonded to N) > CH2NH2 > H. The H needs to be moved to the back position which causes the priority to go to the right (clockwise) which indicates R.
b) S: COOH > CH2OH > C
 CH > H. Since the H is coming forward, you can assign the priority and it goes to the right (clockwise which would be R) but since the lowest priority is forward, you have to switch it to S. Alternatively, you can rotate the molecule to put the lowest priority to the back and you’ll see that it rotates left (or counterclockwise) for S.
CH > H. Since the H is coming forward, you can assign the priority and it goes to the right (clockwise which would be R) but since the lowest priority is forward, you have to switch it to S. Alternatively, you can rotate the molecule to put the lowest priority to the back and you’ll see that it rotates left (or counterclockwise) for S.c) S: Br > OH > NH2 > CH3. Since the lowest priority is going back, you can follow the priority and see that it is going left (counterclockwise) and therefore S.
Exercise \(\PageIndex{8}\)
Draw the structure of (R)-2-bromohexane.
- Answer
-
Exercise \(\PageIndex{9}\)
Draw the structure of (S)-2-methyl-3-pentanol.
- Answer
-
Outside links
References
- Schore and Vollhardt. Organic Chemistry Structure and Function. New York:W.H. Freeman and Company, 2007.
- McMurry, John and Simanek, Eric. Fundamentals of Organic Chemistry. 6th Ed. Brooks Cole, 2006.
Contributors and Attributions
- Ekta Patel (UCD), Ifemayowa Aworanti (University of Maryland Baltimore County)
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
- Dr. Zachary Sharrett (Sonoma State University)
- Layne Morsch (University of Illinois Springfield)

