5.2: Nomenclature
- Page ID
- 402353
Classification of Ligands
Let us look a little closer at ligands and see how we can classify them. One way is to categorize them into monodentate and multi-dentate ligands.
Monodentate ligands have only one point of attachment to the metal ion.
Monodentate ligands have only one point of attachment to the metal ion
Examples for such ligands are halogenide ligands, ammonia as ligand, and water as ligand. The molecules often have different names when acting as ligands, and you must know these names. For example, water as a ligand is called an aqua ligand, ammonia as a ligand is called an ammine ligand, chloride as a ligand is called a chloro ligand.
Ligands with more than one point of attachment are called multidentate ligands, or chelate ligands.
Multi-dentate ligands have two or more points of attachment to the metal ion
Complexes with chelate ligands are called chelate complexes. Greek prefixes indicate how many points of attachment the ligand has (Fig. 5.2.1).

If there are two, then we have a bidentate ligand, when there are three, we have a tridentate ligand. We use the prefixes tetra, penta, and hexa to indicate four, five, and six points of attachment, respectively. Multi-dentate ligands with more than six points of attachment are rare.
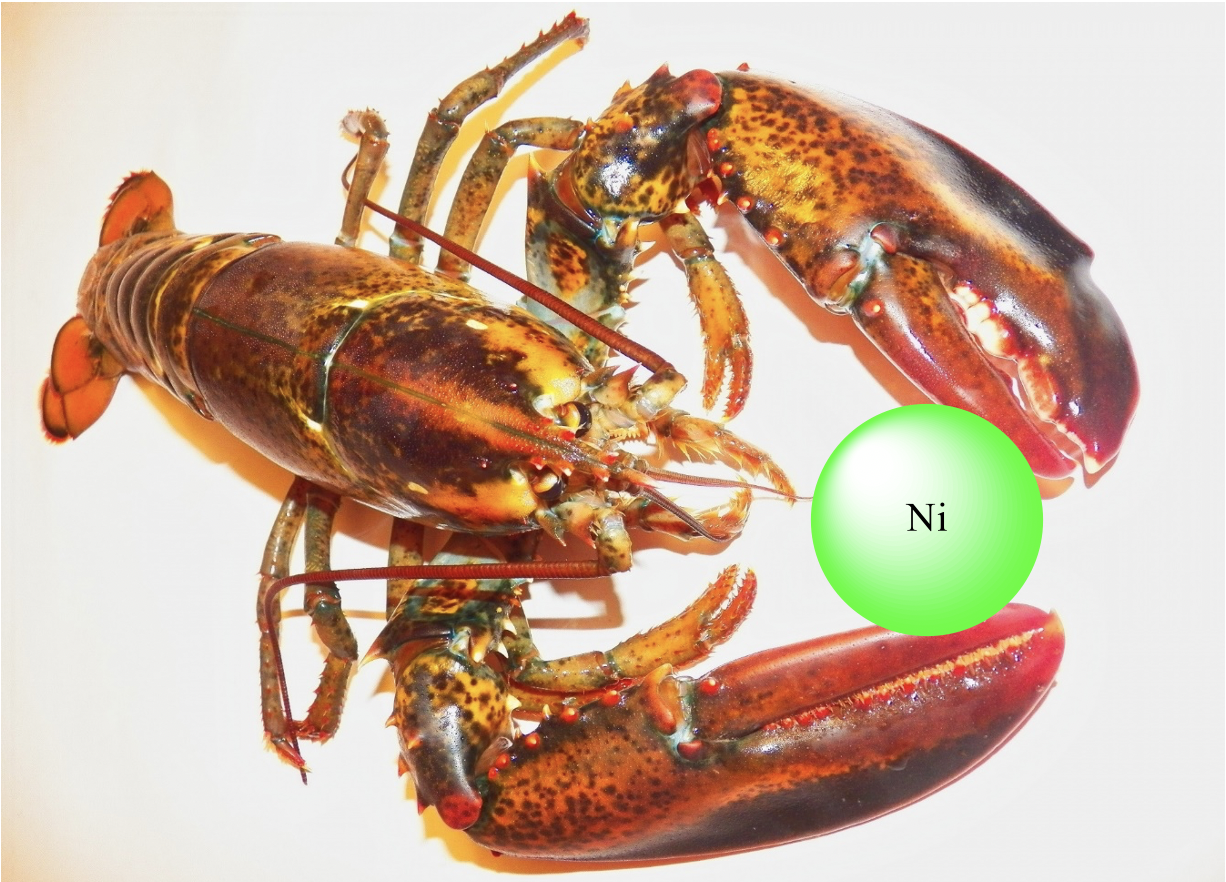
The name chelate ligand comes from the Greek word chela, meaning great claw of the lobster. We can see that our lobster in Figure 5.2.2 happily chelates a Ni2+ ion with its two great claws!
Common Bidentate Ligands
A few common bidentate ligands are shown below.
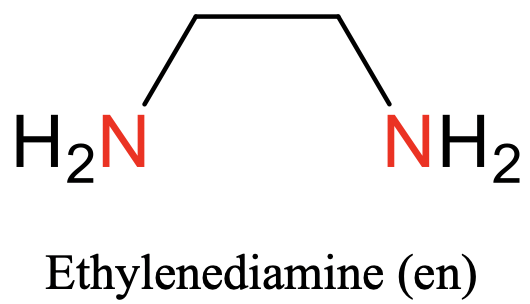
The first one is ethylene diamine. It has two nitrogen donor atoms that can bind to the metal, and they are separated by an ethylene group (Fig. 5.2.3).
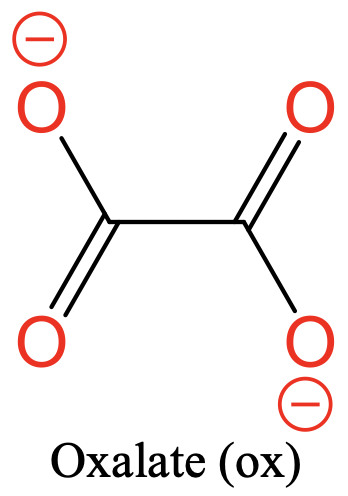
Another common ligand is the oxalate ligand. It has two O donor atoms separated by two carbon atoms. There are two negative charges that are delocalized over the four O atoms (Fig. 5.2.4).
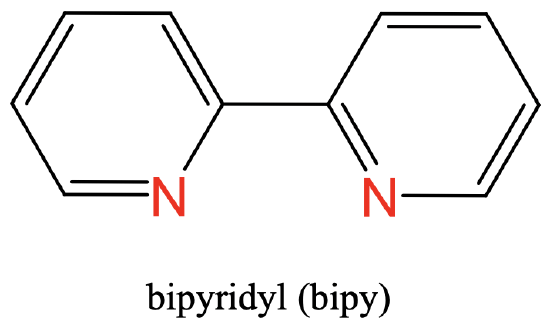
As a third example you can see the bipyridyl ligand having two N donor atoms as part of two aromatic rings. The two N atoms are separated by two carbon atoms (Fig. 5.2.5).
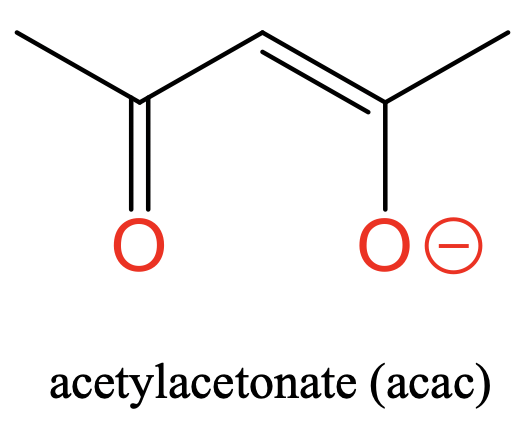
Lastly, there is the acetyl acetonate ligand with two O donor atoms separated by three C atoms (Fig. 5.2.6). The acetyl acetonate carries a negative charge that is delocalized between the two O atoms. In this case the donor atoms are separated by three carbons.
Common ligands often have specific abbreviations. They are often used in the formulas of coordination compounds with these ligands. For example, the ethylene diamine ligand is abbreviated en, the oxalate ligand is abbreviated ox, the bipyridyl ligand is abbreviated bipy, and the acetyl acetonate ligand is abbreviated acac. There are not only bidentate ligands with O and N donor atoms, but also with others such as P and S.
Rings in Complexes with Chelate Ligands
In most chelating ligands the donor atoms are separated by two or three other atoms, mostly carbon atoms. This is because in this case the chelate ligands can form five- and six-membered rings with the metal ion (Fig. 5.2.7).
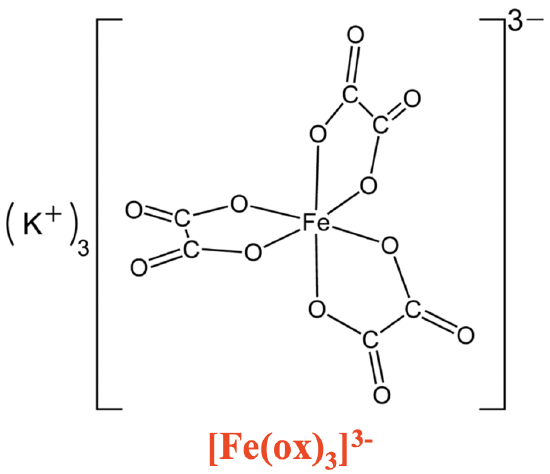
You can see that the trioxolato iron complex above has three five-membered rings containing one Fe, two O, and two C atoms. These ring sizes are particularly stable because they have the least ring strain. As a consequence, the respective chelate complexes are particularly stable.
Tridentate Ligands
Here are a couple of examples for tridentate ligands.
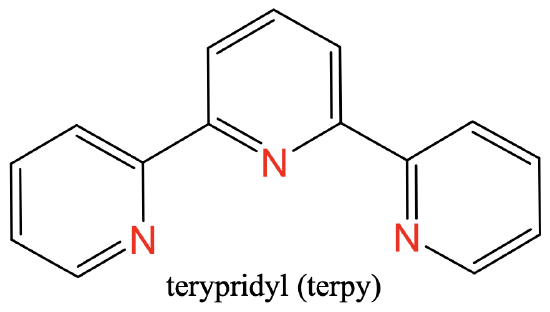
The first one is terpyridyl, abbreviated “Terpy”(Fig. 5.2.8), the second one is bisethylenetriamine, abbreviated “tris” (Fig. 5.2.9).
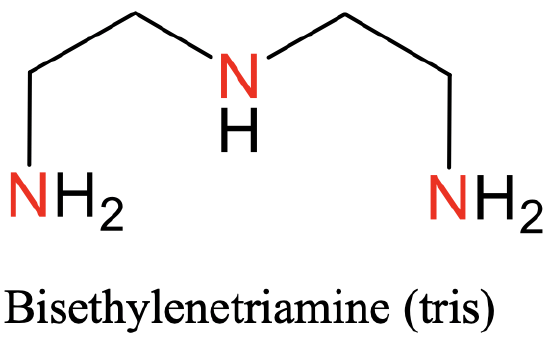
Both of them have three N-donor atoms separated by two carbon atoms. In the “tris”-ligand there are two ethylene groups between the N atoms, in the case of the “terpy”-ligand the N-atoms are part of three aromatic rings (Fig. 5.2.8. and Fig. 5.2.9)
Tetradentate Ligands
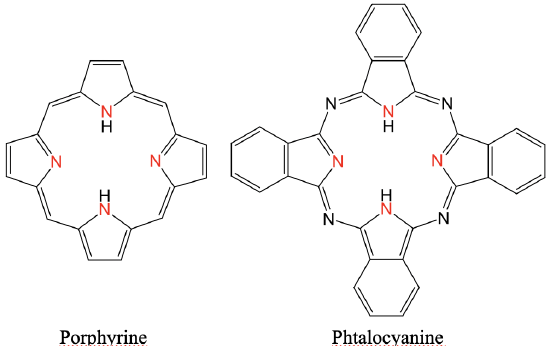
Two common tetradentate ligands are porphyrine and phtalocyanine (Fig. 5.2.9 and 5.2.10). Both are co-called macrocyclic ligands because they are large cycles. They both have four N-donor atoms pointing inside of the cycle. The phtalocyanine ligand has four additional N atoms connecting the five-membered rings via imine-linkages. Further the phtalocyanine has four benzene rings fused to the four five-membered rings. The porphyrine ligand is very important in biological systems. For example, it is part of the protein hemoglobin. In this case a an Fe2+ ion sits in the center of the porphyrin ring. It is also a component of chlorophyll in which case an Mg2+ ion sits in the center of the ring. Phtalocyanine ligands are important as components of dyes.
Hexadentate Ligand, EDTA
A very common hexadentate ligand is the ethylenediamine tetraacetic acid (EDTA) ligand. You can see its structure below (Fig. 5.2.11).
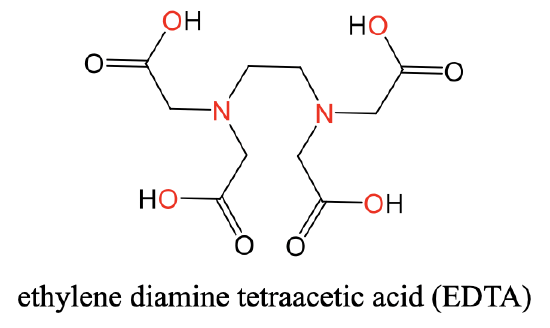
It has two N-donor atoms separated by an ethylene group. Each N-atom is further connected to two acetyl groups. The overall four acetyl groups carry four O-donor atoms. Overall, there are six donor atoms. The six donor atoms can coordinate octahedrally to a metal ion such as a Ca2+ ion (Fig. 5.2.12).
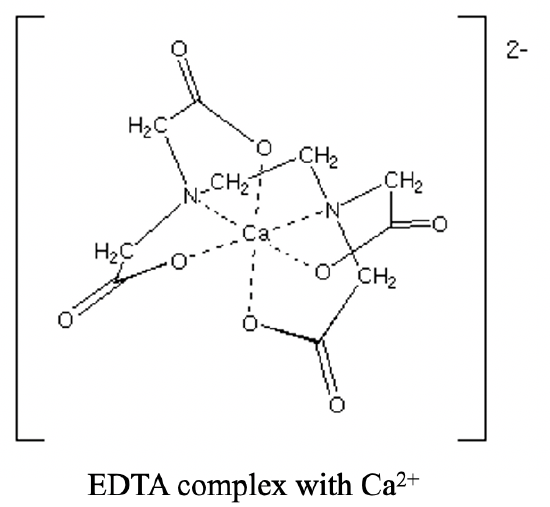
In coordinated form the EDTA ligand is deprotonated, and the O donor atoms carry a negative charge. Therefore an EDTA complex with a divalent cation such as Ca2+ has a 2- charge.
Nomenclature of Complexes
Now let us develop a nomenclature for coordination chemistry so that we can communicate them in an educated manner. One important aspect is that we name the number of ligands. To indicate the number of a particular ligand we use Greek prefixes (Fig. 5.2.13).
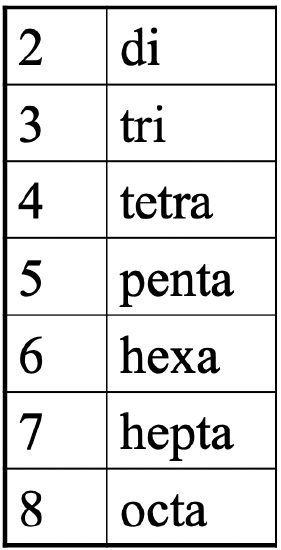
These are the same prefixes we got to know when we discussed multidentate ligands. If there are two ligands we use the prefix di, if there are three we use the prefix tri- and so fourth.
Note that there are a few more rules and guides for nomenclature of these types of compounds. For the time being, this level of detail should be enough to let you put together what type of compound is being discussed.
Dr. Kai Landskron (Lehigh University). If you like this textbook, please consider to make a donation to support the author's research at Lehigh University: Click Here to Donate.


