Homework 15
- Page ID
- 28872
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q 4.95
Question: Arrange the following elements from highest to lowest metallic character: S, Al, Cs, F, Sr, Cl
Solution:
A. In order to solve this problem, one must first know that metallic character increases as you go down a group in the periodic table, and as you go left within a period on the periodic table.
B. Then, in order to determine the metallic character on each of the elements, you have to look at their location on the periodic table.
C. Since the question asks for the elements to be listed in order of decreasing metallic character, it would make more sense to find the element with the highest metallic character first, then the second highest, etc.
D. Double check to make sure they are in the correct order and that you have not skipped any elements.
A. When moving from left to right, metallic character decreases because the elements tend to gain electrons to fill valence shells rather than lose them. Elements on the right side, like chlorine, will gain electrons to fill their valence shells, while elements on the left side, like cesium, will lose electrons in order to have a full valence shell. When moving down the periodic table, metallic character increases because elements can lose electrons more easily as the atomic radius increases. This is because the attraction between the nucleus and valence electrons decreases as there is more distance between them.
B. Pull up a periodic table on a computer, or one that is in your Chemistry book. This is so that you can visually compare where each element is at on the periodic table.
C. Look on the bottom left side of the table and try to locate some of the elements listed. You will see that cesium (Cs) and strontium (Sr) are placed on the bottom left side, so they will have the highest metallic characters. Cesium is farther down and more left than strontium, so cesium will have the highest metallic character and strontium will have the second highest metallic character. Next, you will see that aluminum (Al) is located farther left than sulfur, chlorine, and fluorine. This means that aluminum will be the element with the third highest metallic character. The only elements left are sulfur (S), chlorine (Cl), and fluorine (F). They are all located within a very close distance of one another. Sulfur is the farthest left and farthest down of the three, so it will have the fourth highest metallic character. Chlorine is right below fluorine, making it the element with the fifth highest metallic character. This leaves fluorine as the last element, meaning it has the lowest metallic character.
D. We found cesium, strontium, aluminum, sulfur, chlorine, and fluorine on the periodic table. Cesium is the farthest left and the lowest, while fluorine is the farthest right and the highest, so we know they have the highest metallic character and the lowest metallic character, respectively.
Answer: Cs, Sr, Al, S, Cl, F
Q 6.55
Question: For each figure, state the:
a) total number of electron groups
b) number of bonding pairs
c) number of lone pairs
d) electron geometry
e) molecular geometry
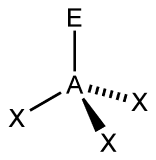
Figure 1:
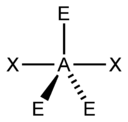
Figure 2:
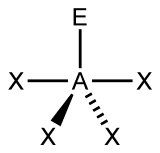
Figure 3:
Solution:
A. Begin with Figure 1, find the total number of electron groups, each line, wedge, or dash represents an electron group. Use this information to determine the electron geometry. Then determine how many bonding pairs it has (X represents bonding pairs). Then, determine how many lone pairs it has (E represents lone pairs). Use this information to determine the molecular geometry.
B. Move on to Figure 2, and follow the same procedure as you did for Figure 1.
C. Move on to Figure 3, and follow the same procedure as you did for Figure 1 and Figure 2.
A. As you can see, Figure 1 appears to have 4 electron groups in total. This gives you the information that Figure 1 has a tetrahedral electron geometry. Tetra is the prefix for 4, so you can use that to assist you in coming to an answer. Since X represents a bonding pair, you can determine that Figure 1 has 3 bonding pairs. There is only one E, so it has one lone pair. This makes the AXE notation AX3E. This information allows you to determine the molecular geometry of the figure. An AXE notation of AX3E means that this figure is trigonal pyramidal. The 3 bonding pairs make the triangular base of the pyramid because there are 3 sides to a triangle, and the one lone pair forms the tip, making it a pyramid.
B. Figure 2 appears to have 5 electron groups in total. This means it has a trigonal bipyramidal electron geometry. There are 2 Xs, meaning it has 2 bonding pairs. There are 3 Es, meaning it has 3 lone pairs. This makes the AXE notation out to be AX2E3. This notation leads us to the conclusion that it has a linear molecular geometry. The two bonding pairs make a line, which is the simpler way of determining they form a linear molecular geometry.
C. Figure 3 has 5 electron groups in total, so we can determine it has a trigonal bipyramidal electron geometry, like Figure 2. This figure has 4 Xs, meaning it has 4 bonding pairs. There is only 1 E, so it only has one lone pair. This makes the AXE notation out to be AX4E. There are many different molecular geometries within the category of trigonal bipyramidal, but this one in particular has 4 bonding pairs and 1 lone pair. This means it has a seesaw molecular geometry. One of the bottom two Xs comes towards the front and one goes towards the back. If you picture the figure in 3D without the lone pair, it would look kind of like a seesaw.
Answer:
Figure 1
a) 4 electron groups
b) 3 bonding pairs
c) 1 lone pair
d) tetrahedral
e) trigonal bipyramidal
Figure 2
a) 5 electron groups
b) 2 bonding pairs
c) 3 lone pairs
d) trigonal bipyramidal
e) linear
Figure 3
a) 5 electron groups
b) 4 bonding pairs
c) 1 lone pair
d) trigonal bipyramidal
e) seesaw