9.6.3: The Strengths of Acids and Bases, and the pH Scale
- Page ID
- 478486
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the difference between strong and weak acids and bases.
- Use Ka values to rank weak acids in order of higher or lower acidity.
- Use the pH scale to describe acids and bases and rank solutions for being acidic, basic, and neutral.
Acids and bases do not all demonstrate the same degree of chemical activity in solution. Different acids and bases have different strengths.
Strong and Weak Acids
Let us consider the strengths of acids first. A small number of acids ionize completely in aqueous solution. For example, when HCl dissolves in water, every molecule of HCl separates into a hydronium ion and a chloride ion:
\[\ce{HCl(g) + H2O(l) ->[\sim 100\%] H_3O^{+}(aq) + Cl^{-} (aq)} \label{Eq1} \]
HCl(aq) is one example of a strong acid, which is a compound that is essentially 100% ionized in aqueous solution. There are very few strong acids. The important ones are listed in Table \(\PageIndex{1}\).
| Acids | Bases |
|---|---|
| HCl | LiOH |
| HBr | NaOH |
| HI | KOH |
| HNO3 | Mg(OH)2 |
| H2SO4 | Ca(OH)2 |
| HClO4 |
By analogy, a strong base is a compound that is essentially 100% ionized in aqueous solution. As with acids, there are only a few strong bases, which are also listed in Table \(\PageIndex{1}\).
If an acid is not listed in Table \(\PageIndex{1}\), it is likely a weak acid, which is a compound that is not 100% ionized in aqueous solution. Similarly, a weak base is a compound that is not 100% ionized in aqueous solution. For example, acetic acid (\(\ce{HC2H3O2}\)) is a weak acid. The ionization reaction for acetic acid is as follows:
\[\ce{HC2H3O2(aq) + H2O(ℓ) \rightarrow H3O^{+}(aq) + C2H3O^{−}2(aq)} \label{Eq2} \]
Depending on the concentration of HC2H3O2, the ionization reaction may occur only for 1%–5% of the acetic acid molecules.
Many household products are acids or bases. For example, the owner of a swimming pool may use muriatic acid to clean the pool. Muriatic acid is another name for hydrochloric acid [\(\ce{HCl(aq)}\)]. Vinegar has already been mentioned as a dilute solution of acetic acid [\(\ce{HC2H3O2(aq)}\)]. In a medicine chest, one may find a bottle of vitamin C tablets; the chemical name of vitamin C is ascorbic acid (\(\ce{HC6H7O6}\)).
One of the more familiar household bases is ammonia (NH3), which is found in numerous cleaning products. As we mentioned previously, ammonia is a base because it increases the hydroxide ion concentration by reacting with water:
\[\ce{NH3(aq) + H2O(ℓ) \rightarrow NH^{+}4(aq) + OH^{−}(aq)} \label{Eq3} \]
Many soaps are also slightly basic because they contain compounds that act as Brønsted-Lowry bases, accepting protons from water and forming excess hydroxide ions. This is one reason that soap solutions are slippery.

Perhaps the most dangerous household chemical is the lye-based drain cleaner. Lye is a common name for sodium hydroxide, although it is also used as a synonym for potassium hydroxide. Lye is an extremely caustic chemical that can react with grease, hair, food particles, and other substances that may build up and form a clog in a pipe. Unfortunately, lye can also attack tissues and other substances in our bodies. Thus, when we use lye-based drain cleaners, we must be very careful not to touch any of the solid drain cleaner or spill the water it was poured into. Safer, nonlye drain cleaners use peroxide compounds to react on the materials in the clog and clear the drain.
Another important safety tip for using household cleaners is to never use bleach with other cleaning products. The cleaning products can react with bleach to produce chlorine gas and chloramines which are toxic and deadly.
Chemical Equilibrium in Weak Acids and Bases
The behavior of weak acids and bases illustrates a key concept in chemistry. Does the chemical reaction describing the ionization of a weak acid or base just stop when the acid or base is done ionizing? Actually, no. Rather, the reverse process—the reformation of the molecular form of the acid or base—occurs, ultimately at the same rate as the ionization process. For example, the ionization of the weak acid \(\ce{HC2H3O2 (aq)}\) is as follows:
\[\ce{HC2H3O2(aq) + H2O(ℓ) \rightarrow H3O^{+}(aq) + C2H3O^{−}2(aq)} \label{Eq4} \]
The reverse process also begins to occur:
\[\ce{H3O^{+}(aq) + C2H3O^{−}2(aq) \rightarrow HC2H3O2(aq) + H2O(ℓ)} \label{Eq5} \]
Eventually, there is a balance between the two opposing processes, and no additional change occurs. The chemical reaction is better represented at this point with a double arrow:
\[\ce{HC2H3O2(aq) + H2O(ℓ) <=> H3O^{+}(aq) + C2H3O^{-}2(aq)} \label{Eq6} \]
The \(\rightleftharpoons\) implies that both the forward and reverse reactions are occurring, and their effects cancel each other out. A process at this point is considered to be at chemical equilibrium (or equilibrium). It is important to note that the processes do not stop. They balance out each other so that there is no further net change; that is, chemical equilibrium is a dynamic equilibrium.
Write the equilibrium chemical equation for the partial ionization of each weak acid or base.
- HNO2(aq)
- C5H5N(aq)
Solution
- HNO2(aq) + H2O(ℓ) ⇆ NO2−(aq) + H3O+(aq)
- C5H5N(aq) + H2O(ℓ) ⇆ C5H5NH+(aq) + OH−(aq)
Write the equilibrium chemical equation for the partial ionization of each weak acid or base.
- \(HF_{(aq)}\)
- \(AgOH_{(aq)}\)
- CH3NH2(aq)
- Answer
-
a. HF(aq) + H2O(ℓ) ⇆ F−(aq) + H3O+(aq)
b. AgOH(aq) ⇆ Ag+(aq) + OH−(aq)
c. CH3NH2(aq) + H2O(ℓ) ⇆ CH3NH3+(aq) + OH−(aq)
Acid Ionization Constant, \(K_\text{a}\)
Chemists have created a rating system that measures the strength of a weak acid. It is based on the ionization reaction for a general weak acid, \(\ce{HA}\):
\[\ce{HA} \left( aq \right) \rightleftharpoons \ce{H^+} \left( aq \right) + \ce{A^-} \left( aq \right) \nonumber \]
The numerical value of \(K_\text{a}\) is a reflection of the strength of the acid. Several examples are shown in the table below. Weak acids with larger \(K_\text{a}\) values are stronger than acids with smaller \(K_\text{a}\) values.
The table below is a listing of acid ionization constants for several acids. Note that polyprotic acids have a distinct ionization constant for each ionization step, with each successive ionization constant being smaller than the previous one.
| Name of Acid | \(K_\text{a}\) Value* |
|---|---|
| Oxalic acid (found in spinach and rhubarb) |
\(5.6 \times 10^{-2}\) |
| Citric acid (in citrus foods) | \(7.1 \times 10^{-4}\) |
| Lactic acid (in dairy foods and fermented foods) | \(1.4 \times 10^{-4}\) |
| Malic acid (found in apples and other fruits and vegetables) | \(4.0 \times 10^{-4}\) |
| Acetic acid (found in vinegar and fermented foods) | \(1.8 \times 10^{-5}\) |
| Carbonic acid (dissolved in water to make carbonated drinks) |
\(4.2 \times 10^{-7}\) |
*Some acids, such as citric acid, malic acid, and carbonic acid, have more than one acidic proton and are polyprotic. In this table, the Ka value for only the most acidic H+ is listed.
Strong and Weak Bases and Base Ionization Constant, Kb
As with acids, bases can either be strong or weak, depending on their extent of ionization. A strong base is a base, which ionizes completely in an aqueous solution. The most common strong bases are soluble metal hydroxide compounds such as potassium hydroxide. Some metal hydroxides are not as strong simply because they are not as soluble. Calcium hydroxide is only slightly soluble in water, but the portion that does dissolve also dissociates into ions.
A weak base is a base that ionizes only slightly in an aqueous solution. Recall that a base can be defined as a substance, which accepts a hydrogen ion from another substance. When a weak base such as ammonia is dissolved in water, it accepts an \(\ce{H^+}\) ion from water, forming the hydroxide ion and the conjugate acid of the base, the ammonium ion.
\[\ce{NH_3} \left( aq \right) + \ce{H_2O} \left( l \right) \rightleftharpoons \ce{NH_4^+} \left( aq \right) + \ce{OH^-} \left( aq \right) \nonumber \]
As with acids, chemists rate the strength of bases based, and use a numerical value of \(K_\text{b}\). Weak bases with relatively higher \(K_\text{b}\) values are stronger than bases with relatively lower \(K_\text{b}\) values. Table \(\PageIndex{3}\) is a listing of base ionization constants for several weak bases.
| Name of Base | \(K_\text{b}\) |
|---|---|
| Methylamine | \(5.6 \times 10^{-4}\) |
| Ammonia | \(1.8 \times 10^{-5}\) |
| Pyridine | \(1.7 \times 10^{-9}\) |
| Acetate ion | \(5.6 \times 10^{-10}\) |
| Fluoride ion | \(1.4 \times 10^{-11}\) |
| Urea | \(1.5 \times 10^{-14}\) |
The Ion-Product of Water
As we have already seen, H2O can act as an acid or a base. Within any given sample of water, some \(\ce{H2O}\) molecules are acting as acids, and other \(\ce{H2O}\) molecules are acting as bases. The chemical equation is as follows:
\[\color{red}{\underbrace{\ce{H2O}}_{\text{acid}}} + \color{blue}{\underbrace{\ce{H2O}}_{\text{base}}} \color{black} \ce{<=> H3O^{+} + OH^{−}} \label{Auto} \]
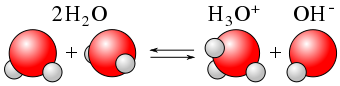
Similar to a weak acid, the autoionization of water is an equilibrium process, and is more properly written as follows:
\[\ce{H2O(ℓ) + H2O(ℓ) <=> H3O^{+}(aq) + OH^{-}(aq)} \label{Eq7} \]
We often use the simplified form of the reaction:
\[\ce{H2O(l) <=> H+(aq) + OH−(aq)} \nonumber \]
The equilibrium constant for the autoionization of water is referred to as the ion-product for water and is given the symbol Kw.
\[K_w = [\ce{H^{+}}][\ce{OH^{-}}] \nonumber \] = 1.0 \times 10^{−14} \nonumber \]
This means that...
- For solutions that are acidic, the concentration of H+ or [H+] is greater than 1.0×10−7 M
- For solutions that are basic, the concentration of OH− or [OH−] is greater than 1.0×10−7 M
- For neutral solutions, [H3O+] = [OH−] = 1.0×10−7M
The pH Scale
One qualitative measure of the strength of an acid or a base solution is the pH scale, which is based on the concentration of the hydronium (or hydrogen) ion in aqueous solution.
\[pH = -\log[H^+] \nonumber \]
or
\[pH = -\log[H_3O^+] \nonumber \]
Figure \(\PageIndex{3}\) illustrates this relationship, along with some examples of various solutions.
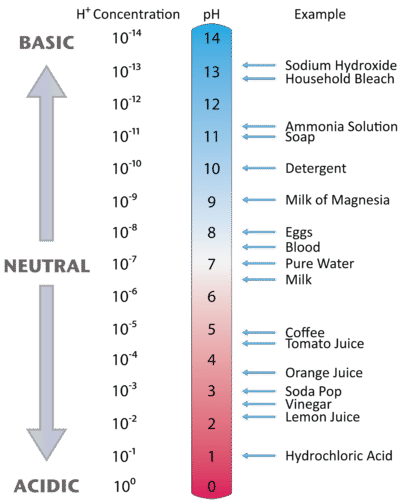
A neutral (neither acidic nor basic) solution has a pH of 7. A pH below 7 means that a solution is acidic, with lower values of pH corresponding to increasingly acidic solutions. A pH greater than 7 indicates a basic solution, with higher values of pH corresponding to increasingly basic solutions. Thus, given the pH of several solutions, you can state which ones are acidic, which ones are basic, and which are more acidic or basic than others. These are summarized in Table \(\PageIndex{4}.
Table \(\PageIndex{4}\): Acidic, Basic and Neutral pH Values
| Classification | Relative Ion Concentrations | pH at 25 °C |
|---|---|---|
| acidic | [H+] > [OH−] | pH < 7 |
| neutral | [H+] = [OH−] | pH = 7 |
| basic | [H+] < [OH−] | pH > 7 |
For each of the following solutions, classify them as acidic, basic, or neutral:
- pickle juice, pH 3.3
- apricot juice, pH 4.0
- drinking water, pH 7.2
Solution
The pH scale ranks items from most acidic (low pH) to most basic (high pH), with pH = 7 for a neutral solution.
Substitute the known quantity into the equation and solve. Use a scientific calculator for b and c.
- acidic (pH is less than 7)
- acidic (pH is less than 7)
- basic (pH is more than 7)
Exercise \(\PageIndex{3}\)
Consider the following solutions:
Olive brine, pH 3.7
Shrimp puree, pH 7.0
Hominy cooking water, pH 7.5
Grapefruit juice, pH 3.1
- Which is the most acidic?
- Which is the most basic?
- Which is neutral?
- Answer
-
a. Grapefruit juice
b. Hominy cooking water
c. Shrimp puree
Table \(\PageIndex{5}\) lists the pH of several common solutions. The most acidic among the listed solutions is battery acid with the lowest pH value (0.3). The most basic is 1M NaOH solution with the highest pH value of 14.0. Notice that some biological fluids (stomach acid and urine) are nowhere near neutral. You may also notice that many food products are slightly acidic. They are acidic because they contain solutions of weak acids. If the acid components of these foods were strong acids, the food would likely be inedible.
| Solution | pH |
|---|---|
| battery acid | 0.3 |
| stomach acid | 1–2 |
| lemon or lime juice | 2.1 |
| vinegar | 2.8–3.0 |
| Coca-Cola | 3 |
| wine | 2.8–3.8 |
| beer | 4–5 |
| coffee | 5 |
| milk | 6 |
| urine | 6 |
| pure H2O | 7 |
| (human) blood | 7.3–7.5 |
| sea water | 8 |
| antacid (milk of magnesia) | 10.5 |
| NH3 (1 M) | 11.6 |
| bleach | 12.6 |
| NaOH (1 M) | 14.0 |
Label each solution as acidic, basic, or neutral based only on the stated \(pH\).
- milk of magnesia, pH = 10.5
- pure water, pH = 7
- wine, pH = 3.0
Solution
- With a pH greater than 7, milk of magnesia is basic. (Milk of magnesia is largely Mg(OH)2.)
- Pure water, with a pH of 7, is neutral.
- With a pH of less than 7, wine is acidic.
Exercise \(\PageIndex{4}\)
Identify each substance as acidic, basic, or neutral based only on the stated \(pH\).
- human blood with \(pH\) = 7.4
- household ammonia with \(pH\) = 11.0
- cherries with \(pH\) = 3.6
- Answer
-
a. slightly basic
b. basic
c. acidic
Key Takeaways
- Acids and bases can be strong or weak depending on the extent of ionization in solution.
- Most chemical reactions reach equilibrium at which point there is no net change.
- The ion-product of [H+][OH−] in an aqueous solution remains constant.
- A pH value is simply the negative of the logarithm of the H+ ion concentration (-log[H+]).
- The pH scale is used to succinctly communicate the acidity or basicity of a solution.
- A solution is acidic if pH < 7.
- A solution is basic if pH > 7.
- A solution is neutral if pH = 7.


