5: Thin-Layer Chromatography (TLC)
- Page ID
- 549327
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Purpose of this Experiment is to:
- Separate analgesics with TLC and compute accurate Rf values.
- Optimize eluent polarity (e.g., hexanes/ethyl acetate) to obtain informative Rf values (≈0.2–0.8).
- Visualize spots using UV first; apply iodine/chemical stains only if needed (stains are typically destructive).
INTRODUCTION
Chromatography techniques operate on the principle that different compounds distribute themselves differently between two phases: a stationary phase and a mobile phase. In TLC, the stationary phase is a thin layer of a solid adsorbent—most commonly silica gel or alumina—coated onto a glass, plastic, or aluminum plate. These materials are polar and interact strongly with polar functional groups in organic molecules.
Thin‑layer chromatography (TLC) is a fast, simple, and widely used analytical technique in organic chemistry that allows chemists to separate, identify, and analyze the components of a mixture. Because TLC requires only very small sample amounts and minimal equipment, it is commonly used in instructional laboratories to study the behavior of organic compounds and to introduce fundamental principles of chromatography.
The mobile phase in TLC is a liquid solvent or solvent mixture, called the eluent. When a TLC plate is placed upright in a developing chamber containing a shallow pool of eluent, the solvent rises up the plate by capillary action. As the solvent travels upward, it carries the sample components with it. However, because each compound interacts differently with the stationary and mobile phases, it moves at different rates, leading to separation.
In normal‑phase TLC (the most common mode used in teaching laboratories), less polar compounds interact weakly with the polar stationary phase and spend more time dissolved in the mobile phase. As a result, they travel farther up the TLC plate. More polar compounds, by contrast, interact more strongly with the stationary phase and move more slowly. This difference in polarity is the primary factor governing separation in TLC.
Once development is complete, the solvent is allowed to evaporate, and the separated compounds appear as distinct spots on the plate. Because many organic compounds are colorless, the spots are typically visualized using a short‑wave ultraviolet (UV) lamp or chemical visualization methods such as iodine vapor or staining solutions. Under UV light, compounds that absorb UV radiation appear as dark spots against a fluorescent background.
The results of a TLC analysis are expressed quantitatively using the retention factor (Rf), or Rf value. The Rf value is defined as the ratio of the distance traveled by a compound to the distance traveled by the solvent front:

The Rf value is always between 0 and 1 and is characteristic of a compound only under a specific set of conditions, including the stationary phase, solvent system, and temperature. For this reason, comparisons between unknown and known compounds must be made using TLC plates developed simultaneously under identical conditions.
Thin‑layer chromatography has many practical applications in the chemistry laboratory. It is commonly used to monitor the progress of chemical reactions, assess the purity of compounds, select appropriate solvent systems for column chromatography, and identify unknown substances by comparison with known standards. In this experiment, TLC will be used in conjunction with melting-point measurements to identify the active ingredient in an unknown analgesic sample.
General Procedure for TLC
1. Spotting.
A pencil line is drawn parallel to the short side of the TLC plate 1.0 cm from the edge. Two or three points evenly spaced are marked on the line. The sample (~1mg) to be analyzed is placed in a 0.1mL conical vial and a few drops of solvent are added. A capillary tube is used to apply a small fraction of the solution from the vial to the TLC plate.
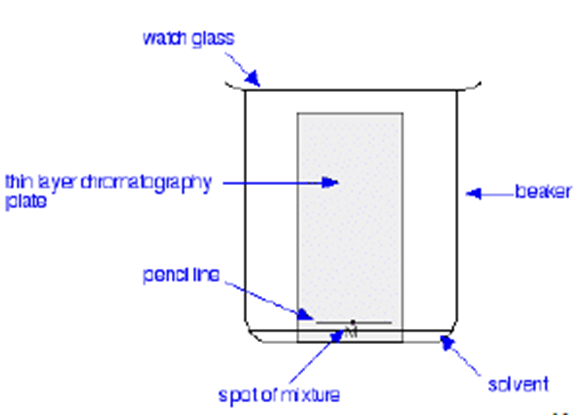
2. Developing
The chromatogram is performed by placing the spotted thin-layer plate in a wide-mouth jar or beaker with a watch glass cover, then adding a small amount of developing solvent. The material spot on the TLC plate initially must be positioned above the solvent line. The jar is quickly recapped or the watch glass replaced in order to maintain an atmosphere saturated with the developing solvent. The elution solvent rapidly ascends the plate by capillary action. The spotted material becomes eluted vertically up the plate. Development is interrupted when the solvent line reaches the top of the plate. The position of the solvent front should be quickly marked on the plate when the chromatogram is terminated.
3. Visualizing
If the compounds being chromatographed are colorless, visualization can be achieved by placing the plate in an iodine vapor chamber after the thin-layer plate has been removed from the developing chamber and allowed to stand for a few minutes. Organic materials readily absorb the iodine vapor to produce brown spots. On removal from the iodine chamber, the spots are marked by pencil because they will rapidly fade. The second widely used method is by placing the plate under an ultraviolet light. Since spots disappear when the light is removed, it is necessary to circle the spots with a pencil in order to have a permanent record of the chromatogram.
Once the separation of the components of the mixture is complete and the individual spots have been detected, the retention factor (Rf) of each compound may be calculated as shown below:
The Rf value for a compound is a physical constant for a given set of chromatographic conditions, so the adsorbent and the eluting solvent should be recorded along with the experimentally determined Rf values.
In this experiment, you will determine the Rf values of some commercially available analgesics or other compounds (identified by your instructor when conducting the experiment) and identify an unknown analgesic.


