9.4 Hydration of Alkynes
- Page ID
- 44329
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- write the equation for the reaction of water with an alkyne in the presence of sulphuric acid and mercury(II) sulphate.
- describe keto-enol tautomerism.
- predict the structure of the ketone formed when a given alkyne reacts with sulphuric acid in the presence of mercury(II) sulfate.
- identify the reagents needed to convert a given alkyne to a given ketone.
- identify the alkyne needed to prepare a given ketone by hydration of the triple bond.
- write an equation for the reaction of an alkyne with borane.
- write the equation for the reaction of a vinylic borane with basic hydrogen peroxide or hot acetic acid.
- identify the reagents, the alkyne, or both, needed to prepare a given ketone or a given cis alkene through a vinylic borane intermediate.
- identify the ketone produced when a given alkyne is reacted with borane followed by basic hydrogen peroxide.
- identify the cis alkene produced when a given alkyne is reacted with borane followed by hot acetic acid.
- explain why it is necessary to use a bulky, sterically hindered borane when preparing vinylic boranes from terminal alkynes.
- predict the product formed when the vinylic borane produced from a terminal alkyne is treated with basic hydrogen peroxide.
- identify the alkyne needed to prepare a given aldehyde by a vinylic borane.
Key Terms
Make certain that you can define, and use in context, each of the key terms listed below.
- enol
- keto-enol tautomeric equilibrium (see the “Study Notes,” below)
- tautomerism (see the “Study Notes,” below)
- tautomers
Study Notes
Rapid interconversion between tautomers is called tautomerism; however, as the two tautomers are in equilibrium, the term tautomeric equilibrium may be used. This section demonstrates the equilibrium between a ketone and an enol; hence, the term keto-enol tautomeric equilibrium is appropriate. The term “enol” indicates the presence of a carbon-carbon double bond and a hydroxyl (i.e., alcohol) group. Later in the course, you will see the importance of keto-enol tautomerism in discussions of the reactions of ketones, carbohydrates and nucleic acids. It is important to note that tautomerism is not restricted to keto-enol systems. Other examples include imine-enamine tautomerism
and nitroso-oxime tautomerism
However, at the moment you need only concern yourself with keto-enol tautomerism. Notice how hydroboration complements hydration in the chemistry of both alkenes and alkynes.
Reaction: Hydration of Alkynes
As with alkenes,hydration (addition of water) to alkynes requires a strong acid, usually sulfuric acid, and is facilitated by mercuric sulfate. However, unlike the additions to double bonds which give alcohol products, addition of water to alkynes gives ketone products ( except for acetylene which yields acetaldehyde ). The explanation for this deviation lies in enol-keto tautomerization, illustrated by the following equation. The initial product from the addition of water to an alkyne is an enol (a compound having a hydroxyl substituent attached to a double-bond), and this immediately rearranges to the more stable keto tautomer.
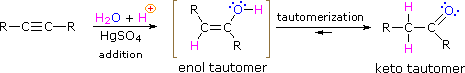
Tautomers are defined as rapidly interconverted constitutional isomers, usually distinguished by a different bonding location for a labile hydrogen atom (colored red here) and a differently located double bond. The equilibrium between tautomers is not only rapid under normal conditions, but it often strongly favors one of the isomers ( acetone, for example, is 99.999% keto tautomer ). Even in such one-sided equilibria, evidence for the presence of the minor tautomer comes from the chemical behavior of the compound. Tautomeric equilibria are catalyzed by traces of acids or bases that are generally present in most chemical samples. The three examples shown below illustrate these reactions for different substitutions of the triple-bond. The tautomerization step is indicated by a red arrow. For terminal alkynes the addition of water follows the Markovnikov rule, as in the second example below, and the final product ia a methyl ketone ( except for acetylene, shown in the first example ). For internal alkynes ( the triple-bond is within a longer chain ) the addition of water is not regioselective. If the triple-bond is not symmetrically located ( i.e. if R & R' in the third equation are not the same ) two isomeric ketones will be formed.
| HC≡CH + H2O + HgSO4 & H2SO4 ——> [ H2C=CHOH ] ——> H3C-CH=O |
| RC≡CH + H2O + HgSO4 & H2SO4 ——> [ RC(OH)=CH2 ] ——> RC(=O)CH3 |
| RC≡CR' + H2O + HgSO4 & H2SO4 ——> [ RHC=C(OH)R' + RC(OH)=CHR' ] ——> RCH2-C(=O)R' + RC(=O)-CH2R' |
With the addition of water, alkynes can be hydrated to form enols that spontaneously tautomerize to ketones. Reaction is catalyzed by mercury ions. Follows Markovnikov’s Rule: Terminal alkynes give methyl ketones
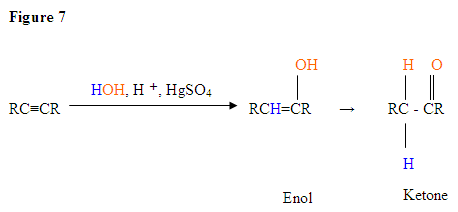
- The first step is an acid/base reaction where the
π electrons of the triple bond acts as a Lewis base and attacks the proton therefore protinating the carbon with the most hydrogen substituents. - The second step is the attack of the nucleophilic water molecule on the electrophilic carbocation, which creates an oxonium ion.
- Next you deprotonate by a base, generating an alcohol called an enol, which then tautomerizes into a ketone.
- Tautomerism is a simultaneous proton and double bond shift, which goes from the enol form to the keto isomer form as shown above in Figure 7.
Now let's look at some Hydration Reactions.
Hydration of Terminal Alkyne produces methyl ketones
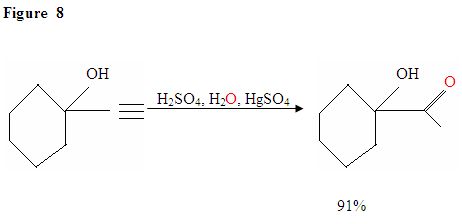
Just as described in Figure 7 the
Hydration of Alkyne
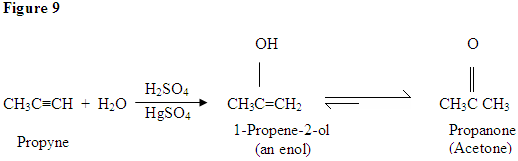
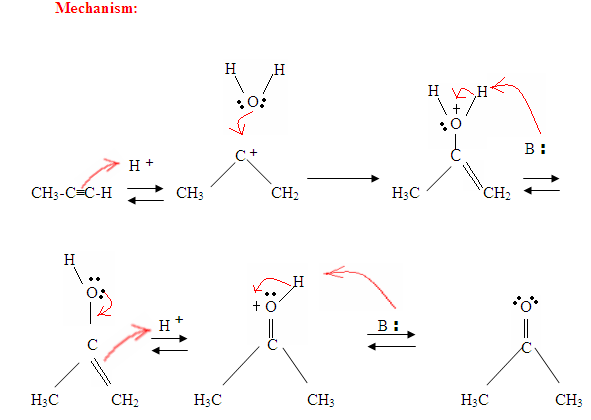
As you can see here, the
Tautomerism is shown here when the proton gets attacked by the double bond
Problems
1. Draw the structure of the product formed when each of the substances below is treated with H2O/H2SO4 in the presence of HgSO4.
2. Draw the structure of the keto form of the compound shown below.
Which form would you expect to be the most stable?
3.
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

