8.8 Oxidation of Alkenes: Cleavage to Carbonyl Compounds
- Page ID
- 44317
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objective
After completing this section, you should be able to
- write an equation to describe the cleavage of an alkene by ozone, followed by reduction of the ozonide so formed with either sodium borohydride or zinc and acetic acid.
- predict the products formed from the ozonolysis-reduction of a given alkene.
- write an equation to describe the cleavage of an alkene by potassium permanganate.
- predict the products from the oxidative cleavage of a given alkene by potassium permanganate.
- use the results of ozonolysis-reduction, or cleavage with permanganate, to deduce the structure of an unknown alkene.
- identify the reagents that should be used in the oxidative cleavage of an alkene to obtain a given product or products.
- write the equation for the cleavage of a 1,2-diol by periodic acid, and draw the structure of the probable intermediate.
- predict the product or products that will be formed from the treatment of a given 1,2-diol with periodic acid.
- use the results of hydroxylation/1,2-diol cleavage to deduce the structure of an unknown alkene.
Key Terms
Make certain that you can define, and use in context, each of the key terms listed below.
- molozonide (see the “Study Notes,” below)
- ozonide
- ozonolysis (see the “Study Notes,” below)
Study Notes
Ozonolysis, or ozonolysis-reduction, refers to the treatment of an alkene with ozone followed by a suitable reducing agent to break down complex double-bond-containing compounds into smaller, more easily identified products. From the identity of the products formed, it may be possible to deduce the structure of the original double-bond-containing substance. Ozonolysis will feature prominently in many of the road-map problems that you will encounter in this course. A molozonide is an unstable, cyclic intermediate that is initially formed when an alkene reacts with ozone. Do not worry about the names used for some of the compounds you encounter in this section. At this point, you are responsible only for the nomenclature of alkanes and alkenes, and their halogenated derivatives. Diol cleavage is another example of a redox reaction; periodic acid, HIO4, is reduced to iodic acid, HIO3.
Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (\(O_3\)), a reactive allotrope of oxygen. The process allows for carbon-carbon double or triple bonds to be replaced by double bonds with oxygen. This reaction is often used to identify the structure of unknown alkenes. by breaking them down into smaller, more easily identifiable pieces. Ozonolysis also occurs naturally and would break down repeated units used in rubber and other polymers. On an industrial scale, azelaic acid and pelargonic acids are produced from ozonolysis.
.jpg?revision=1&size=bestfit&width=476&height=116)
Introduction
The gaseous ozone is first passed through the desired alkene solution in either methanol or dichloromethane. The first intermediate product is an ozonide molecule which is then further reduced to carbonyl products. This results in the breaking of the Carbon-Carbon double bond and is replaced by a Carbon-Oxygen double bond instead.
Reaction Mechanism
Step 1:
.jpg?revision=1&size=bestfit&width=462&height=145)
The first step in the mechanism of ozonolysis is the initial electrophilic addition of ozone to the Carbon-Carbon double bond, which then form the molozonide intermediate. Due to the unstable molozonide molecule, it continues further with the reaction and breaks apart to form a carbonyl and a carbonyl oxide molecule.
Step 2:
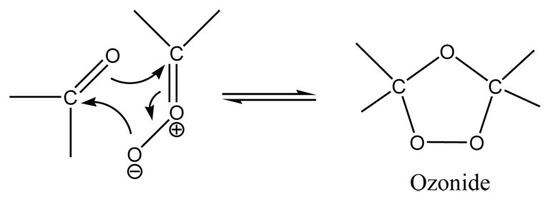
The carbonyl and the carbonyl oxide rearranges itself and reforms to create the stable ozonide intermediate. A reductive workup could then be performed to convert convert the ozonide molecule into the desired carbonyl products.
References
- Vollhardt, K., Schore, N. Organic Chemistry: Structure and Function. 5th ed. New York, NY: W. H. Freeman and Company, 2007.
- Shore, N. Study Guide and Solutions Manual for Organic Chemistry. 5th ed. New York, NY: W.H. Freeman and Company, 2007.
Problems
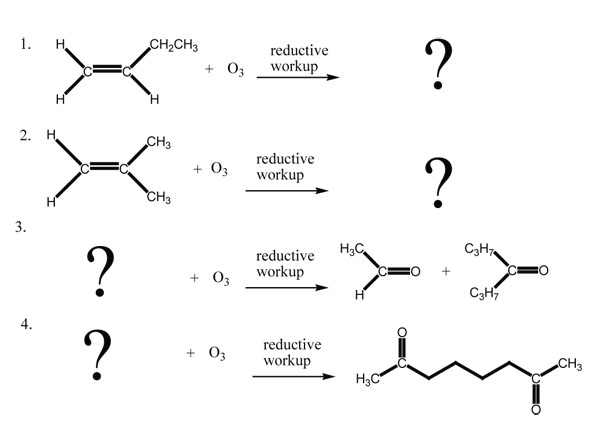
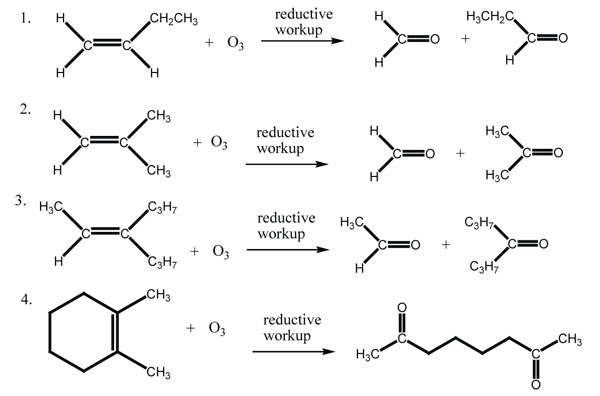
Answers
Exercises
2. What important point did you learn from questions 1(a) and 1(b), above?
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."

