8.4 Hydration of Alkenes: Addition of \(H_{2}O\) by Oxymercuration
- Page ID
- 44313
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- write an equation for the hydration of an alkene with sulphuric acid.
- write an equation for the formation of an alcohol from an alkene by the oxymercuration-demercuration process.
- identify the alkene, the reagents, or both, that should be used to produce a given alcohol by the oxymercuration-demercuration process.
- write the mechanism for the reaction of an alkene with mercury(II) acetate in aqueous tetrahydrofuran (THF).
Key Terms
Make certain that you can define, and use in context, each of the key terms listed below.
- hydration
- oxymercuration (see the “Study Notes,” below)
Study Notes
Oxymercuration is the reaction of an alkene with mercury(II) acetate in aqueous THF, followed by reduction with sodium borohydride. The final product is an alcohol. It is important that you recognize the similarity between the mechanisms of bromination and oxymercuration. Recognizing these similarities helps you to reduce the amount of factual material that you need to remember. Yet another abbreviation has been introduced on page 271 of the textbook: mercuric acetate, or mercury(II) acetate, to give it the preferred IUPAC name, is written as Hg(OAc)2; by comparing this formula with the formula Hg(O2CCH3)2, you can equate Ac with OCCH3. In fact, Ac is an abbreviation used for the acetyl group with the structure shown below.
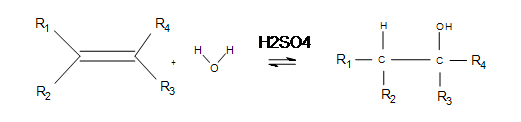
Electrophilic hydration is the act of adding electrophilic hydrogen from a non-nucleophilic strong acid (a reusable catalyst, examples of which include sulfuric and phosphoric acid) and applying appropriate temperatures to break the alkene's double bond. After a carbocation is formed, water bonds with the carbocation to form a 1º, 2º, or 3º alcohol on the alkane.
What Is Electrophilic Hydration?
Electrophilic hydration is the reverse dehydration of alcohols and has practical application in making alcohols for fuels and reagents for other reactions. The basic reaction under certain temperatures (given below) is the following:
The phrase "electrophilic" literally means "electron loving" (whereas "nucleophilic" means "nucleus loving"). Electrophilic hydrogen is essentially a proton: a hydrogen atom stripped of its electrons. Electrophilic hydrogen is commonly used to help break double bonds or restore catalysts (see SN2 for more details).
How Does Electrophilic Hydration Work?
Mechanism for 3º Alcohol (1º and 2º mechanisms are similar):
Temperatures for Types of Alcohol Synthesis
Heat is used to catalyze electrophilic hydration; because the reaction is in equilibrium with the dehydration of an alcohol, which requires higher temperatures to form an alkene, lower temperatures are required to form an alcohol. The exact temperatures used are highly variable and depend on the product being formed.
- Primary Alcohol: Less than 170ºC
- Secondary Alcohol: Less than 100ºC
- Tertiary Alcohol: Less than 25ºC
But...Why Does Electrophilic Hydration Work?
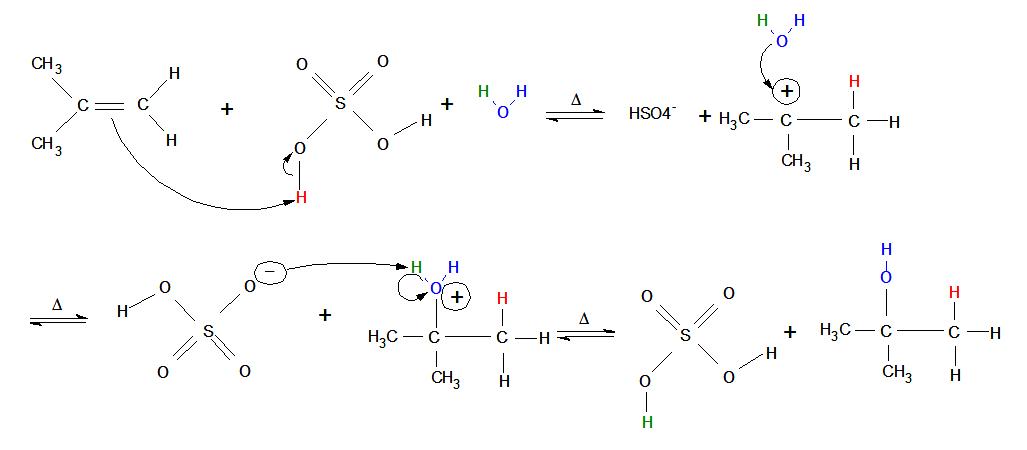
- An alkene placed in an aqueous non-nucleophilic strong acid immediately "reaches out" with its double bond and attacks one of the acid's hydrogen atoms (meanwhile, the bond between oxygen and hydrogen performs heterolytic cleavage toward the oxygen—in other words, both electrons from the oxygen/hydrogen single bond move onto the oxygen atom).
- A carbocation is formed on the original alkene (now alkane) in the more-substituted position, where the oxygen end of water attacks with its 4 non-bonded valence electrons (oxygen has 6 total valence electrons because it is found in Group 6 on the periodic table and the second row down: two electrons in a 2s-orbital and four in 2p-orbitals. Oxygen donates one valence electron to each bond it forms, leaving four 4 non-bonded valence electrons).
- After the blue oxygen atom forms its third bond with the more-substituted carbon, it develops a positive charge (3 bonds and 2 valence electrons give the blue oxygen atom a formal charge of +1).
- The bond between the green hydrogen and the blue oxygen undergoes heterolytic cleavage, and both the electrons from the bond move onto the blue oxygen. The now negatively-charged strong acid picks up the green electrophilic hydrogen.
- Now that the reaction is complete, the non-nucleophilic strong acid is regenerated as a catalyst and an alcohol forms on the most substituted carbon of the current alkane. At lower temperatures, more alcohol product can be formed.
What is Regiochemistry and How Does It Apply?
Regiochemistry deals with where the substituent bonds on the product. Zaitsev's and Markovnikov's rules address regiochemistry, but Zaitsev's rule applies when synthesizing an alkene while Markovnikov's rule describes where the substituent bonds onto the product. In the case of electrophilic hydration, Markovnikov's rule is the only rule that directly applies. See the following for an in-depth explanation of regiochemistry Markovnikov explanation: Radical Additions--Anti-Markovnikov Product Formation
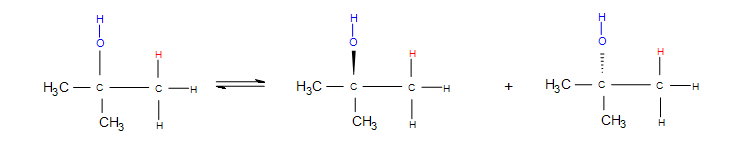
In the mechanism for a 3º alcohol shown above, the red H is added to the least-substituted carbon connected to the nucleophilic double bonds (it has less carbons attached to it). This means that the carbocation forms on the 3º carbon, causing it to be highly stabilized by hyperconjugation—electrons in nearby sigma (single) bonds help fill the empty p-orbital of the carbocation, which lessens the positive charge. More substitution on a carbon means more sigma bonds are available to "help out" (by using overlap) with the positive charge, which creates greater carbocation stability. In other words, carbocations form on the most substituted carbon connected to the double bond. Carbocations are also stabilized by resonance, but resonance is not a large factor in this case because any carbon-carbon double bonds are used to initiate the reaction, and other double bonded molecules can cause a completely different reaction.
If the carbocation does originally form on the less substituted part of the alkene, carbocation rearrangements occur to form more substituted products:
- Hydride shifts: a hydrogen atom bonded to a carbon atom next to the carbocation leaves that carbon to bond with the carbocation (after the hydrogen has taken both electrons from the single bond, it is known as a hydride). This changes the once neighboring carbon to a carbocation, and the former carbocation becomes a neighboring carbon atom.
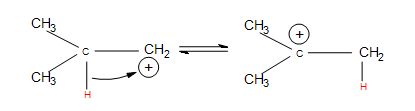
- Alkyl shifts: if no hydrogen atoms are available for a hydride shift, an entire methyl group performs the same shift.
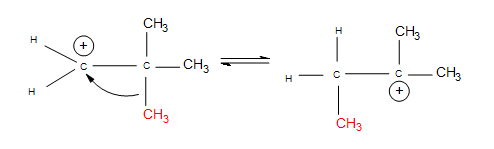
The nucleophile attacks the positive charge formed on the most substituted carbon connected to the double bond, because the nucleophile is seeking that positive charge. In the mechanism for a 3º alcohol shown above, water is the nucleophile. When the green H is removed from the water molecule, the alcohol attached to the most substituted carbon. Hence, electrophilic hydration follows Markovnikov's rule.
What is Stereochemistry and How Does It Apply?
Stereochemistry deals with how the substituent bonds on the product directionally. Dashes and wedges denote stereochemistry by showing whether the molecule or atom is going into or out of the plane of the board. Whenever the bond is a simple single straight line, the molecule that is bonded is equally likely to be found going into the plane of the board as it is out of the plane of the board. This indicates that the product is a racemic mixture.
Electrophilic hydration adopts a stereochemistry wherein the substituent is equally likely to bond pointing into the plane of the board as it is pointing out of the plane of the board. The 3º alcohol product could look like either of the following products:
Note: Whenever a straight line is used along with dashes and wedges on the same molecule, it could be denoting that the straight line bond is in the same plane as the board. Practice with a molecular model kit and attempting the practice problems at the end can help eliminate any ambiguity.
Is this a Reversible Synthesis?
Electrophilic hydration is reversible because an alkene in water is in equilibrium with the alcohol product. To sway the equilibrium one way or another, the temperature or the concentration of the non-nucleophilic strong acid can be changed. For example:
- Less sulfuric or phosphoric acid and an excess of water help synthesize more alcohol product.
- Lower temperatures help synthesize more alcohol product.
Is There a Better Way to Add Water to Synthesize an Alcohol From an Alkene?
A more efficient pathway does exist: see Oxymercuration - Demercuration: A Special Electrophilic Addition. Oxymercuration does not allow for rearrangements, but it does require the use of mercury, which is highly toxic. Detractions for using electrophilic hydration to make alcohols include:
- Allowing for carbocation rearrangements
- Poor yields due to the reactants and products being in equilibrium
- Allowing for product mixtures (such as an (R)-enantiomer and an (S)-enantiomer)
- Using sulfuric or phosphoric acid
Oxymercuration is a special electrophilic addition. It is anti-stereospecific and regioselective. Regioselectivity is a process in which the substituents choses one direction it prefers to be attached to over all the other possible directions. The good thing about this reaction is that there are no carbocation rearrangement due to stabilization of the reactive intermediate. Similar stabilization is also seen in bromination addition to alkenes.
Introduction
Carbocation rearrangement is a process in which the carbocation intermediate can form a more stable ion. With carbocation rearrangement, the reaction would not be able to hydrate quickly under mild conditions and be produced in high yields. This reaction is very fast and proceeds with 90% yield.
This reaction involves a mercury acting as a reagent attacking the alkene double bond to form a Mercurinium Ion Bridge. A water molecule will then attack the most substituted carbon to open the mercurium ion bridge, followed by proton transfer to solvent water molecule.
The organomercury intermediate is then reduced by sodium borohydride - the mechanism for this final step is beyond the scope of our discussion here. Notice that overall, the oxymercuration - demercuration mechanism follows Markovnikov's Regioselectivity with the OH group is attached to the most substituted carbon and the H is attach to the least substituted carbon. The reaction is useful, however, because strong acids are not required, and carbocation rearrangements are avoided because no discreet carbocation intermediate forms.
References
- Vollhardt, K. Peter C. Organic chemistry structure and function. New York: W.H. Freeman, 2007.
- Smith, Michael B., and Jerry March. March's Advanced Organic Chemistry Reactions, Mechanisms, and Structure (March's Advanced Organic Chemistry). New York: Wiley-Interscience, 2007 2007.
- Roderic P. Quirk , Robert E. Lea, Reductive demercuration of hex-5-enyl-1-mercuric bromide by metal hydrides. Rearrangement, isotope effects, and mechanism, J. Am. Chem. Soc., 1976, 98 (19), pp 5973–5978.
Some Practice Problems
What are the end products of these reactants?
Answers
The end product to these practice problems are pretty much very similar. First, you locate where the double bond is on the reactant side. Then, you look at what substituents are attached to each side of the double bond and add the OH group to the more substituent side and the hydrogen on the less substituent side.
Problems
Predict the product of each reaction.
1)
.bmp?revision=1&size=bestfit&width=326&height=100)
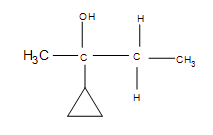
2) How does the cyclopropane group affect the reaction?
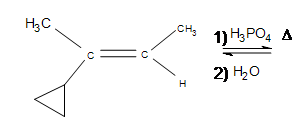
3) (Hint: What is different about this problem?)
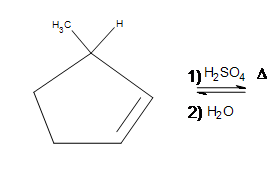
4) (Hint: Consider stereochemistry.)
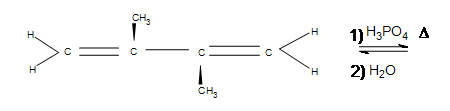
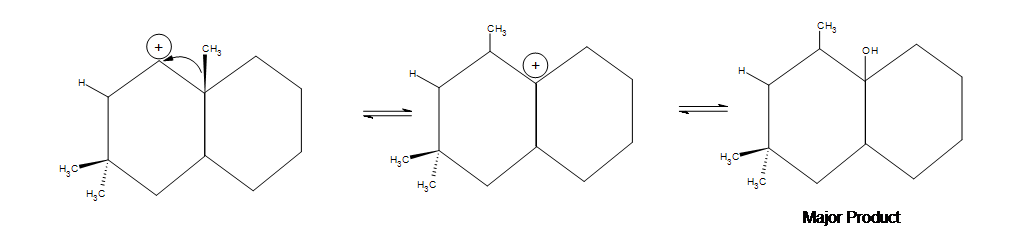
5) Indicate any shifts as well as the major product:

Answers to Practice Problems
1) This is a basic electrophilic hydration.
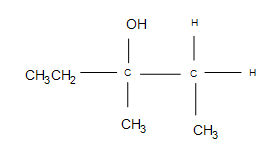
2) The answer is additional side products, but the major product formed is still the same (the product shown). Depending on the temperatures used, the cyclopropane may open up into a straight chain, which makes it unlikely that the major product will form (after the reaction, it is unlikely that the 3º carbon will remain as such).
3) A hydride shift actually occurs from the top of the 1-methylcyclopentane to where the carbocation had formed.
.bmp?revision=1&size=bestfit&width=290&height=201)
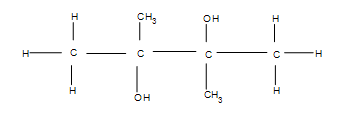
4) This reaction will have poor yields due to a very unstable intermediate. For a brief moment, carbocations can form on the two center carbons, which are more stable than the outer two carbons. The carbocations have an sp2 hybridization, and when the water is added on, the carbons change their hybridization to sp3. This makes the methyl and alcohol groups equally likely to be found going into or out of the plane of the paper- the product is racemic.
5) In the first picture shown below, an alkyl shift occurs but a hydride shift (which occurs faster) is possible. Why doesn't a hydride shift occur? The answer is because the alkyl shift leads to a more stable product. There is a noticeable amount of side product that forms where the two methyl groups are, but the major product shown below is still the most significant due to the hyperconjugation that occurs by being in between the two cyclohexanes.
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Lance Peery (UCD), Duyen Dao-Tran
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
Jim Clark (Chemguide.co.uk)
Prof. Steven Farmer (Sonoma State University)