9.5: Molecular Solids
- Page ID
- 33939
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Discuss the properties of molecular crystals.
A molecule is defined as a discrete aggregate of atoms bound together sufficiently tightly by directed covalent forces to allow it to retain its individuality when the substance is dissolved, melted, or vaporized. The two words italicized in the preceding sentence are important. Covalent bonding implies that the forces acting between atoms within the molecule (intramolecular) are much stronger than those acting between molecules (intermolecular), The directional property of covalent bonding gives each molecule a distinctive shape which affects a number of its properties.
Liquids and solids composed of molecules are held together by van der Waals (or intermolecular) forces, and many of their properties reflect this weak binding. Molecular solids tend to be soft or deformable, have low melting points, and are often sufficiently volatile to sublime directly into the gas phase. This latter property often gives such solids a distinctive odor. Whereas the characteristic melting point of metals and ionic solids is ~1000 °C, most molecular solids melt well below ~300 °C.
Physical Properties of Ionic vs. Covalent Compounds
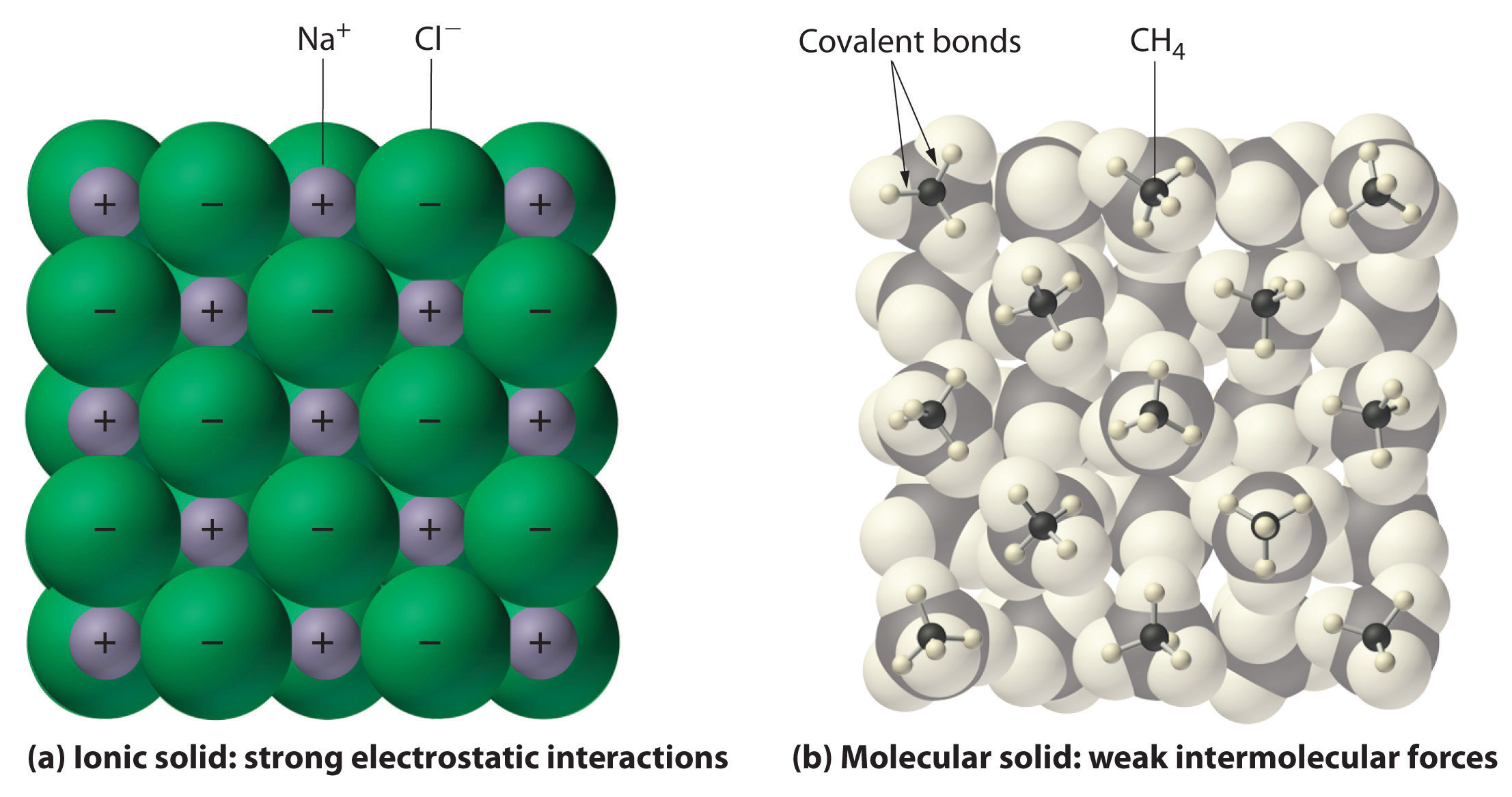
In general, ionic and covalent compounds have different physical properties. Ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. These properties stem from the characteristic internal structure of an ionic solid, illustrated schematically in part (a) in Figure 2.7.4 which shows the three-dimensional array of alternating positive and negative ions held together by strong electrostatic attractions. In contrast, as shown in part (b) in Figure 9.5.1 most covalent compounds consist of discrete molecules held together by comparatively weak intermolecular forces (the forces between molecules), even though the atoms within each molecule are held together by strong intramolecular covalent bonds (the forces within the molecule). Covalent substances can be gases, liquids, or solids at room temperature and pressure, depending on the strength of the intermolecular interactions. Covalent molecular solids tend to form soft crystals that melt at low temperatures and evaporate easily.
Figure 9.5.1: Interactions in Ionic and Covalent Solids. (a) The positively and negatively charged ions in an ionic solid such as sodium chloride (NaCl) are held together by strong electrostatic interactions. (b) In this representation of the packing of methane (\(CH_4\)) molecules in solid methane, a prototypical molecular solid, the methane molecules are held together in the solid only by relatively weak intermolecular forces, even though the atoms within each methane molecule are held together by strong covalent bonds.
Hydrocarbons
Molecular solids consist of atoms or molecules held to each other by dipole–dipole interactions, London dispersion forces, or hydrogen bonds, or any combination of these. The arrangement of the molecules in solid benzene is as follows:
Figure 9.5.2: The structure of solid benzene. In solid benzene, the molecules are not arranged with their planes parallel to one another but at 90° angles.
Because the intermolecular interactions in a molecular solid are relatively weak compared with ionic and covalent bonds, molecular solids tend to be soft, low melting, and easily vaporized (\(ΔH_{fus}\) and \(ΔH_{vap}\) are low). For similar substances, the strength of the London dispersion forces increases smoothly with increasing molecular mass. For example, the melting points of benzene (C6H6), naphthalene (C10H8), and anthracene (C14H10), with one, two, and three fused aromatic rings, are 5.5°C, 80.2°C, and 215°C, respectively. The enthalpies of fusion also increase smoothly within the series: benzene (9.95 kJ/mol) < naphthalene (19.1 kJ/mol) < anthracene (28.8 kJ/mol). If the molecules have shapes that cannot pack together efficiently in the crystal, however, then the melting points and the enthalpies of fusion tend to be unexpectedly low because the molecules are unable to arrange themselves to optimize intermolecular interactions. Thus toluene (C6H5CH3) and m-xylene [m-C6H4(CH3)2] have melting points of −95°C and −48°C, respectively, which are significantly lower than the melting point of the lighter but more symmetrical analog, benzene.
Self-healing rubber is an example of a molecular solid with the potential for significant commercial applications. The material can stretch, but when snapped into pieces it can bond back together again through reestablishment of its hydrogen-bonding network without showing any sign of weakness. Among other applications, it is being studied for its use in adhesives and bicycle tires that will self-heal.
Figure 9.5.3: Toluene and m-xylene. The methyl groups attached to the phenyl ring in toluene and m-xylene prevent the rings from packing together as well as in solid benzene.
Iodine
Iodine is a volatile molecular crystal. In crystals, iodine molecules \(I_2\) are bound by dispersion forces. Due to their weak nature, iodine crystals generate easily visible purple vapor with a strong smell.

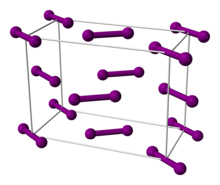
Figure 9.5.4: (Left) Iodine molecular solid and vapor. (Right) Ball-and-stick model of the unit cell of iodine. Images used with permission from wikipedia.
Molecular solids also have relatively low density and hardness. The elements involved are light, and the intermolecular bonds are relatively long and are therefore weak. Because dispersion forces and the other van der Waals forces increase with the number of atoms, large molecules are generally less volatile, and have higher melting points than smaller ones. Also, as one moves down a column in the periodic table, the outer electrons are more loosely bound to the nucleus, increasing the polarizability of the atom, and thus its propensity to van der Waals interactions. This effect is particularly apparent in the increase in boiling points of the successively heavier halogen elements (Table 9.5.1). This increase in size means an increase in the strength of the van der Waals forces.
| Halogen | Melting Point (˚C) | Boiling Point (˚C) |
| Fluorine | -220 | -188 |
| Chlorine | -101 | -35 |
| Bromine | -7.2 | 58.8 |
| Iodine | 114 | 184 |
| Astatine | 302 | 337 |
The vast majority of molecular solids can be attributed to organic compounds containing carbon and hydrogen, such as hydrocarbons (CnHm). Spherical molecules consisting of different number of carbon atoms, called fullerenes, are another important class. Less numerous, yet distinctive molecular solids are halogens (e.g., Cl2) and their compounds with hydrogen (e.g., HCl), as well as light chalcogens (e.g., O2) and pnictogens (e.g., N2).
Key Terms
- intermolecular force: Any of the attractive interactions that occur between atoms or molecules in a sample of a substance.
- Van der Waals force: Attractive forces between molecules (or between parts of the same molecule). These include interactions between partial charges (hydrogen bonds and dipole-dipole interactions), and weaker London dispersion forces
- molecular solid: A solid composed of molecules held together by van der Waals intermolecular forces.
Summary
A molecular solid is composed of molecules held together by van der Waals forces. Its properties are dictated by the weak nature of these intermolecular forces. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (F, Cl, etc.), chalcogens (O, S, etc.), and pnictogens (N, P, etc.). Larger molecules are less volatile and have higher melting points because their dispersion forces increase with the larger number of atoms. Decrease in binding of outer electrons to the nucleus also increases van der Waals-type interactions of the atom due to its increased polarisability.
References
- Wikipedia