An electrocyclic reaction is the concerted cyclization of a conjugated π-electron system by converting one π-bond to a ring forming σ-bond. The key sigma bond must be formed at the terminus of a pi system. These reactions classified by the number of pi electrons involved. Thus, 4 pi reactions form 4 membered rings, as in a conjugated diene can being converted into a cyclobutene. Also, 6 pi reactions form 6 membered rings as in a conjugated triene can be converted into a cyclohexadiene. These reactions are often reversible with the reverse reaction may be called electrocyclic ring opening. Although more more pi electrons can be used, the 4 pi and 6 pi variants are by far the most common and are illustrated below with the key sigma bond highlighted in magenta.

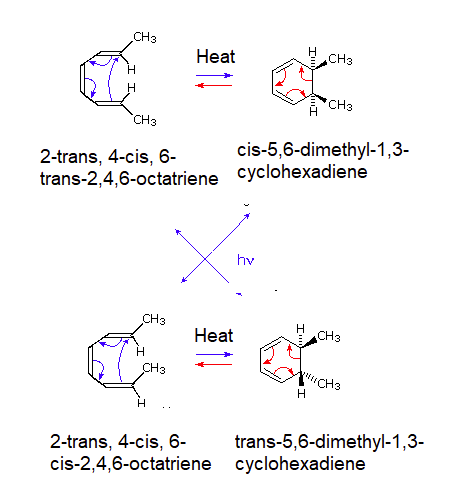
A striking feature of electrocyclic reactions that proceed by concerted mechanisms is their high degree of stereospecificity. For example, 2-trans, 4-cis, 6-trans-2,4,6-octatriene undergoes ring closure to cis-5,6-dimethyl-1,3-cyclohexadiene under thermal conditions i.e. when heated. Similarly the isomeric 2-trans, 4-cis, 6-cis-2,4,6-octatriene produces trans-5,6-dimethyl-1,3-cyclohexadiene, as noted below. However these results are completely reversed if the reaction is run under photochemical conditions (Irradiation with ultraviolet light). For example if 2-trans, 4-cis, 6-cis-2,4,6-octatriene is irradiated with UV light cis-5,6-dimethyl-1,3-cyclohexadiene would be produced.
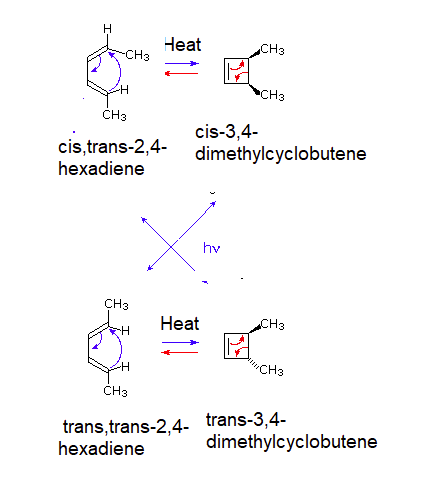
Similar results are seen with the 4 pi electrocyclic reaction of cis,trans-2,4-hexadiene being heated to exclusively form cis-3,4-dimethylcyclobutene and being irradiated with UV light to exclusively form trans-3,4-dimethylcyclobutene. Likewise, trans,trans-2,4-hexadiene forms trans-3,4-dimethylcyclobutene when heated and cis-3,4-dimethylcyclobutene when being irradiated with UV light.
The stereospecificity of electrocyclic reaction can be explained by considering the terminal lobes of the molecular orbital fo the conjugated π-electron system. For electrocyclic reactions of occur, molecular orbital lobes with the same sign from the HOMO of the molecule must rotate to form/break the key ring sigma bond. If the orbital lobes involved both rotate in the same direction (both counterclockwise or both clockwise), the process is called conrotatory. This typically occurs when orbital lobes of the same sign are on opposite sides of the molecule.

If the orbitals involved rotate in opposite directions (one clockwise and one counterclockwise), the process is called disrotatory. These differences in rotation are critically important when stereocenters are formed or broken. This typically occurs when orbital lobes of the same sign are on the same side of the molecule.




