6.12: DEPT ¹³C NMR Spectroscopy
- Page ID
- 452041
After completing this section, you should be able to
- Understand why the peaks do not have splitting like in 1H NMR.
- Using DEPT, distinguish whether a methyl (CH3), methylene (CH2), methine (CH) or quarternary C is present in the molecule and how many.
- Propose a structure based on 13C spectral data.
Distortions Enhancement by Polarization Transfer (DEPT)
DEPT experiments are used for distinguishing between a CH3 group (methyl), a CH2 group (methylene), and a CH group (methine). The proton pulse is set at 45°, 90°, or 135° in the three separate experiments. The different pulses depend on the number of protons attached to a carbon atom. Fig 11. is an example about DEPT spectrum.

Fig \(\PageIndex{1}\). DEPT spectrum of n-isobutlybutrate
While broadband decoupling results in a much simpler spectrum, useful information about the presence of neighboring protons is lost. However, another modern NMR technique called DEPT (Distortionless Enhancement by Polarization Transfer) allows us to determine how many hydrogens are bound to each carbon. For example, a DEPT experiment tells us that the signal at 171 ppm in the ethyl acetate spectrum is a quaternary carbon (no hydrogens bound, in this case a carbonyl carbon), that the 61 ppm signal is from a methylene (CH2) carbon, and that the 21 ppm and 14 ppm signals are both methyl (CH3) carbons. The details of the DEPT experiment are beyond the scope of this text, but DEPT information will often be provided along with 13C spectral data in examples and problems.
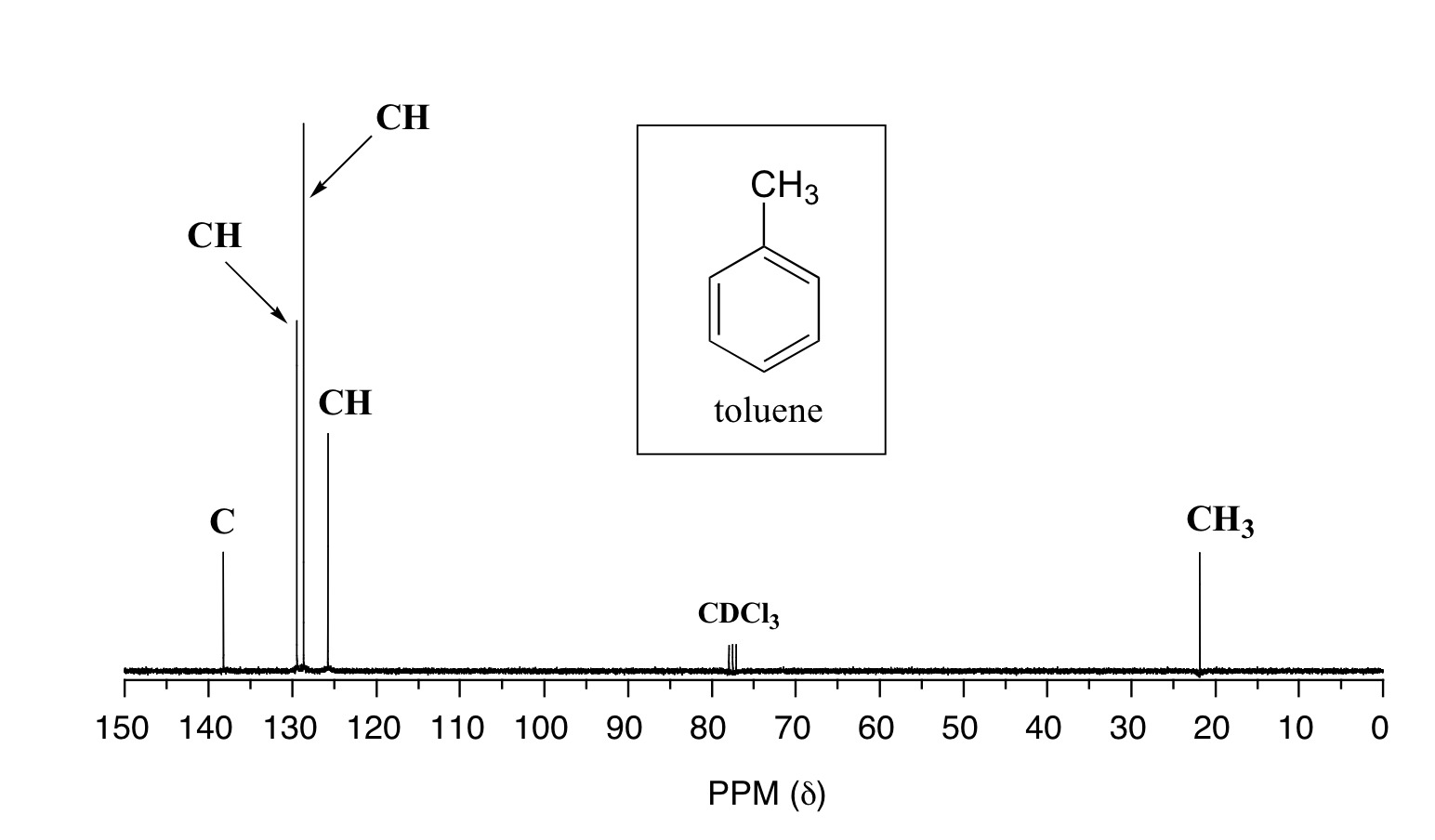
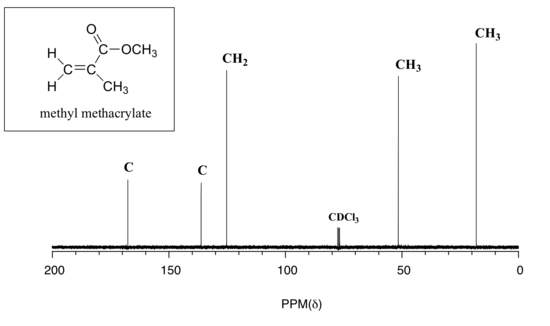
Below are two more examples of 13C NMR spectra of simple organic molecules, along with the type of substitution for that carbon which was obtained from a DEPT experiment.
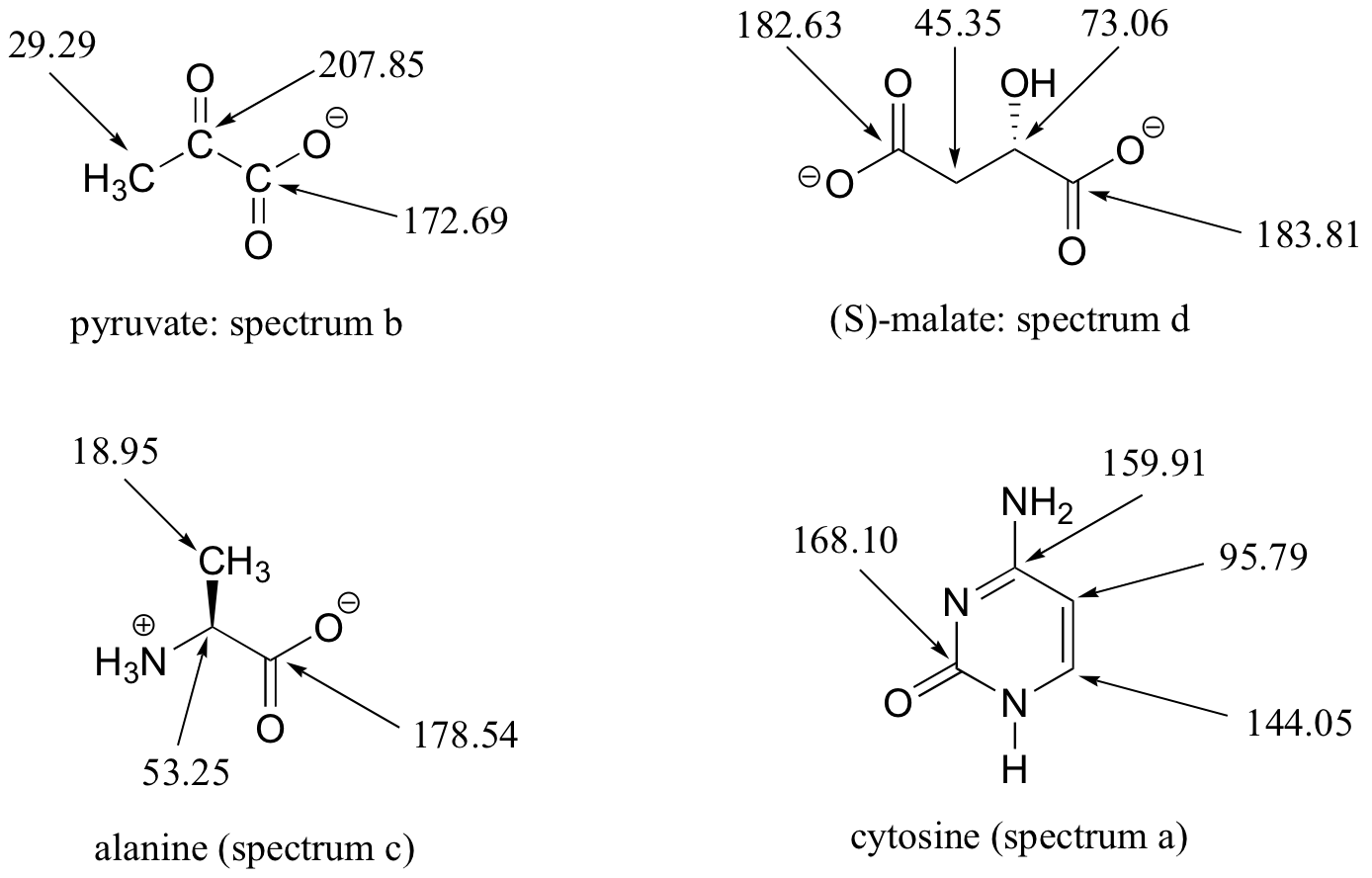
13C-NMR (and DEPT) data for some common biomolecules are shown below (data is from the Aldrich Library of 1H and 13C NMR). Match the NMR data to the correct structure, and make complete peak assignments.
- spectrum a: 168.10 ppm (C), 159.91 ppm (C), 144.05 ppm (CH), 95.79 ppm (CH)
- spectrum b: 207.85 ppm (C), 172.69 ppm (C), 29.29 ppm (CH3)
- spectrum c: 178.54 ppm (C), 53.25 ppm (CH), 18.95 ppm (CH3)
- spectrum d: 183.81 ppm (C), 182. 63 ppm (C), 73.06 ppm (CH), 45.35 ppm (CH2)
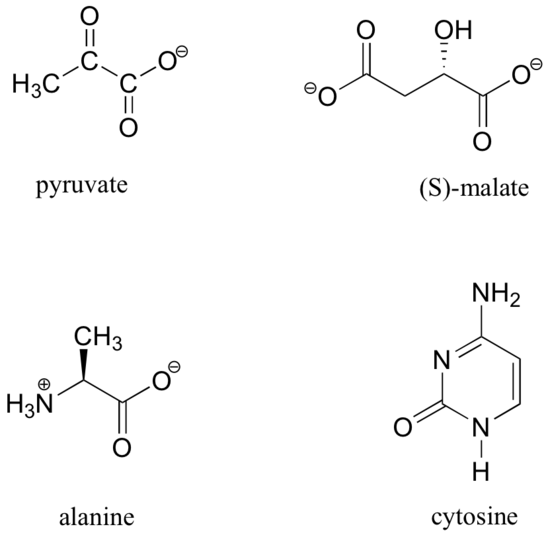
- Answer
-