3.9: Hybridized Orbital Energy Diagrams
- Page ID
- 489173
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Mixing Hybridized Orbitals and Formation of Antibonding orbitals
As shown above in Figure \(\PageIndex{1}\), the energy levels of hybridized atomic orbitals are a weighted average of the mixed atomic orbitals. When two hybridized atoms approach each other, the orbitals mix to form bonds, as shown in Figure \(\PageIndex{2}\) and Figure \(\PageIndex{4}\). However, as with hybridization, mixing of orbitals results in the exact same number of mixed orbitals as there were originally. This means that for every bonding orbital between atoms, there is an antibonding orbital, which is not shown in the diagrams above, for the sake of simplicity. Bonding orbitals are lower in energy than the starting atomic orbitals, and the corresponding antibonding orbitals are higher in energy.
Bonding interactions with a large amount of spatial overlap will be the strongest (e.g., σ bonds) and those with less spatial overlap will be weaker (e.g., π bonds). Each interaction between hybridized orbitals will create an in-phase bonding interaction and an out-of-phase antibonding interaction. For more on bonding, antibonding, and phases of orbitals, see Chapter 3.5: Molecular Orbital Theory.
Hybridized orbital energy levels will vary based on the number of s and p orbitals that are hybridized. For example, sp hybridized orbitals have 50% "s-character" and 50% "p-character" and thus their energy will be halfway between the starting s and p orbital energies, and thus lower in energy than an sp2 orbital on the same element.
In the same way that we can use hybridized atoms to form bonds through overlapping hybridized orbitals in Figure \(\PageIndex{4}\), we can form bonds between the orbitals using energy diagrams to gain insight into the energy levels of different bonds.
Draw the hybridized orbital energy diagram of the C-C bond in ethene (C2H4). What is the lowest energy unoccupied orbital in this diagram?
Solution
1. The Lewis structure of ethene shows a double bond between the two carbons and single bonds with the four Hs.
2. The carbons in ethene are both sp2 hybridized, which gives us one un-hybridized p orbital on each carbon atom and three sp2 orbitals. One of the hybridized orbitals on each carbon is involved in σ bonding (stronger interaction, lower energy bonding orbital) and the un-hybridized p orbital on each carbon is involved in π bonding (weaker interaction). These interactions can be shown in the following diagram:

Example \(\PageIndex{1}\) mix to form bonding interactions and antibonding interactions in molecular orbitals. These molecular orbitals have different energies based on the overlap between the starting hybridized orbitals. The σ bond is formed between two sp2 hybridized orbitals, which point towards each other, as shown in Figure \(\PageIndex{2}\). This strong overlap in atomic orbitals results in a lower energy and more stable bonding orbital. The two p orbitals have less spatial overlap, and thus the bond is less strong between these atomic orbitals, and the corresponding bonding orbital is higher in energy. The stabilization gained in each bonding interaction is proportional to the destabilization of the antibonding orbitals.
Using hybridized orbital energy diagrams for bonding in HCN and H2C=NH,
A) What are the hybridizations of each C and N?
B) Which lone pair is highest in energy between these two molecules?
- Answer
-
First, we need to draw the Lewis structures of each molecule, as shown below.

Then, we can assign their hybridizations based on the Lewis structures (Solution to A):

The hybridizations and Lewis structures will allow us to assign starting energies to the hybridized atomic orbitals:
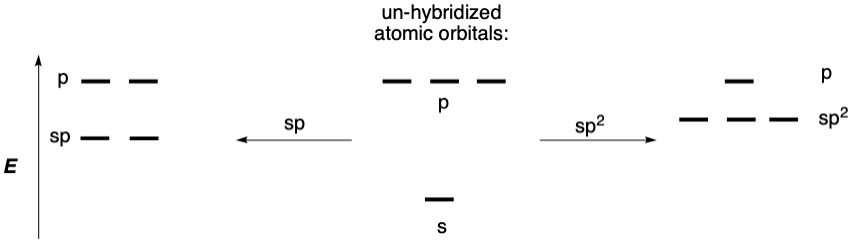
Using these starting energies and the bonding shown in the Lewis structure, we can construct two hybridized orbital energy diagrams.

Now, using the hybridization of the N atoms in each diagram, we can see that an sp hybridized lone pair will be lower in energy than an sp2 hybridized lone pair. Thus, the higher energy lone pair will be the lone pair in H2C=NH.
Note 1: The nitrogen orbitals start lower in energy than the carbon orbitals, because N is more electronegative than C.
Note 2: The nitrogen orbitals are further apart than the carbon orbitals, because the s orbitals are more affected by electronegativity than p orbitals, which leads to a larger s/p orbital difference.

