12.2.2: Predicting UV-visible Absorption
- Page ID
- 281096
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A note from Dr. Haas: The Inorganic Spectroscopy Tutorial from the University of Alberta is an excellent resource for brushing up on Inorganic Spectroscopies. Conveniently, it has a tutorial on UV-visible spectroscopy for Inorganic complexes. Please review this tutorial before reading the page below.
Types of transitions related to the metal ion:
- d-d transitions: d-d transitions are electronic transitions that occur between the molecular orbitals (MOs) that are mostly metal in character; specifically the orbitals that we think of as the d-orbitals of a transition metal complex. These transitions are useful in determining the energy of splitting and can be used to indicate coordination chemistry (geometry and ligand sets). In octahedral complexes, d-d transitions occur between the \(t_{2g}\) and \(e_g\) orbitals (across \(\Delta\)). These transitions cannot occur in metal complexes where the d-orbital is completely empty (\(d^0\)) or completely full (\(d^{10}\)). In other words, a d-d transition is only possible in \(d^1 - d^9\) metal ions. In a UV-visible absorption spectrum, d-d transitions appear as relatively weak absorption with extinction coefficients (\(\varepsilon\)) less than 1,000.
- Charge transfer (CT) transitions: Charge transfer transitions occur between MOs that are mostly metal in character and those that are mostly ligand in character. These transitions depend on the type of ligand: they occur only when the metal is bound to ligands that are \(\pi\)-donors or \(\pi\)-acceptors. And there are two types of CT transitions. If the metal is bound to a \(\pi\)-donor ligand, electrons from lower-energy MO's that are mostly ligand in character can become excited to MO's that are mostly metal in character. These are ligand to metal charge transfers (LMCT) transitions (Figure \(\PageIndex{1}\), left diagram). If the the metal is bound to ligands that are \(\pi\)-acceptors, electrons from the MO's that are mostly metal in character can become excited to higher-energy orbitals that are mostly ligand in character. These are metal to ligand charge transfer (MLCT) transitions (Figure \(\PageIndex{1}\), right diagram). In a UV-visible absorption spectrum, CT transitions appear as relatively intense absorptions with extinction coefficients (\(\varepsilon\)) much greater than 1,000.
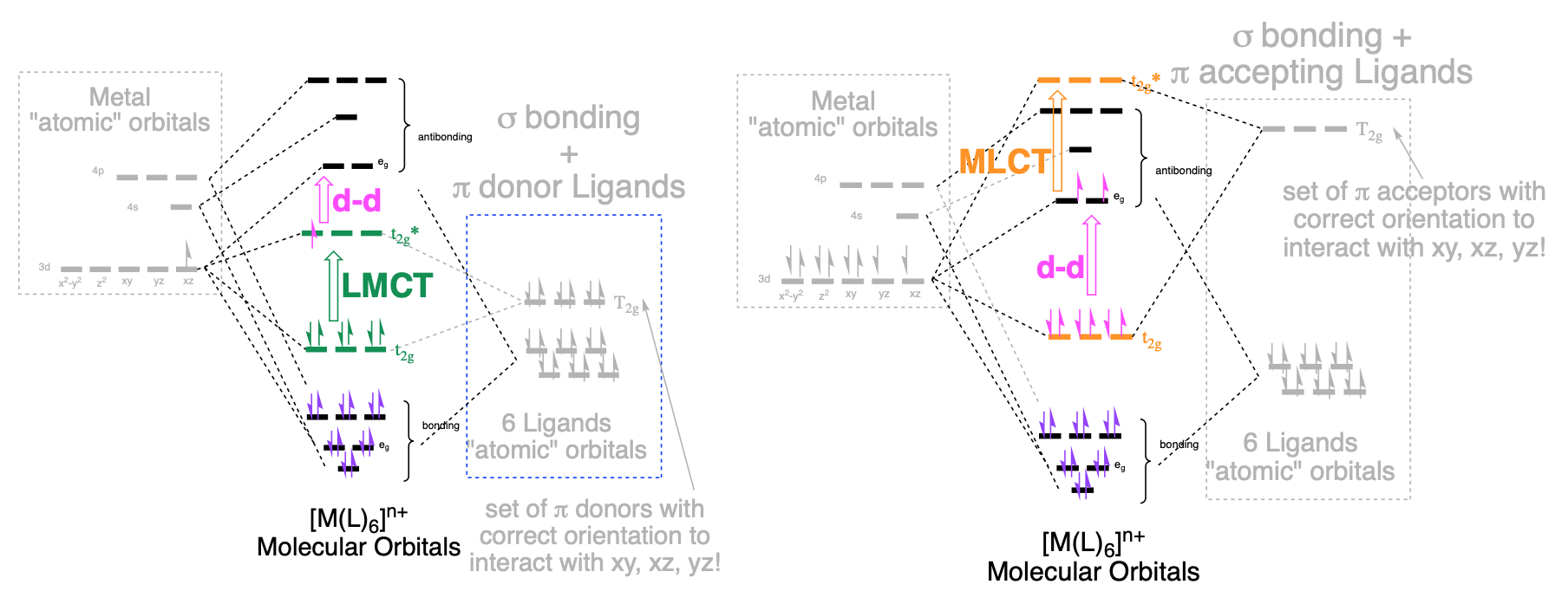
Figure \(\PageIndex{1}\): Abbreviated and annotated MO diagrams for example cases of metal ions in octahdral geometry with sets of \(\pi\)-donor and \(\pi\)-acceptor lignads. In both example MO diagrams, d-d transitions are shown (pink, open arrow from \(t_{2g}\) to \(e_g\)). Left: LMCT transitions are possible with \(\pi\)-donor ligands when electrons are excited from MOs that are mostly ligand in character to MOs that are mostly metal in character. An example of LMCT is shown (green, open arrow from \(t_{2g}\) to \(t*_{2g}\)). Right: MLCT transitions are possible with \(\pi\)-acceptor ligands when electrons from MOs that are mostly metal in character are excited to orbitals that are mostly ligand in character (the \(\pi^*\) orbitals from ligands). An example MLCT is shown ((orange, open arrow from \(e_{g}\) to \(t*_{2g}\)).
Attribution
Curated or created by Kathryn Haas


