9.11: p\(K_\text{a}\)
- Page ID
- 289755
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Calculating p\(K_\text{a}\)
The table below shows some weak acids and their respective \(K_\text{a}\) and p\(K_\text{a}\) values. While we know that the larger the \(K_\text{a}\) value, the stronger the acid, it is difficult to compare the strengths of acids with their \(K_\text{a}\) values since the values are so small. To solve this problem, we use p\(K_\text{a}\), the negative log function of the \(K_\text{a}\) value.
\[pK_a = -log(K_a)\]
\[K_a = 10^{-pK_a}\]
Looking at the table below, we can see that the \(K_\text{a}\) values are all very small, making it difficult to understand which of the acids are stronger. By calculating the p\(K_\text{a}\), we can more easily understand the strengths of the acids and how they compare to each other. p\(K_\text{a}\) is very similar to pH in that the smaller the p\(K_\text{a}\) value, the stronger the acid.
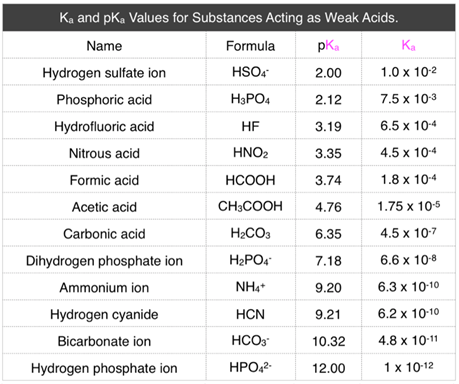
Table \(\PageIndex{1}\): \(K_\text{a}\) and p\(K_\text{a}\) Values of Weak Acids
The Relationship between pH and p\(K_\text{a}\)
Since pH and p\(K_\text{a}\) are calculated the same way we can place p\(K_\text{a}\) on the pH scale to better understand the acidity of a given solution. The most important difference between pH and p\(K_\text{a}\) is that pH is the measure of [H3O+] in a solution, while p\(K_\text{a}\) is specific to an acid. That being said, the pH of a solution can change, while the p\(K_\text{a}\) of an acid will remain constant. Looking at the figure below, the p\(K_\text{a}\) value, 4.76, will not change for the given reaction. The pH, however, changes depending on [H3O+]. We can use the relationship between pH and p\(K_\text{a}\) to understand whether the acidic or basic form of the given reaction is dominating the solution. If we were to shift the pH below the p\(K_\text{a}\), the overall [H3O+] increases. As [H3O+] increases, the equilibrium shifts to the left, which increases the acidic form of the acid. On the other hand, if we were to shift the pH above the p\(K_\text{a}\), indicating a decrease in [H3O+], the equilibrium would shift to the right, increasing the basic form of the acid.
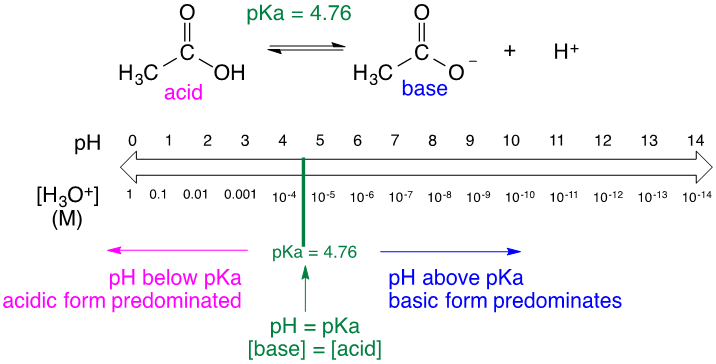
Figure \(\PageIndex{1}\): Reaction and pH scale of acetic acid
So we have demonstrated that pH can indicate if an acid is in its acidic or basic form, but what if the acid contains multiple functional groups? Amino acids can have more than one functional group, and these functional groups have different p\(K_\text{a}\) values. Figure 2 below shows the protonation of alanine, an amino acid. Alanine has two functional groups: an amine (p\(K_\text{a}\)=9.7) and a carboxylic acid (p\(K_\text{a}\)=2.3). Let's start by looking at the carboxylic acid. The carboxylic acid has a p\(K_\text{a}\) of 2.3, meaning it is a stronger acid than the amine. If we place this on the pH scale, we can see for a pH lower than 2.3, the carboxylic acid will be in its acidic form, while for a pH above 2.3, the carboxylic acid will be in its basic form, which is a carboxylate. This is demonstrated by the pink arrows on the pH scale below. Now let's focus on the amine. The amine has a p\(K_\text{a}\) of 9.7, which is much higher than carboxylic acid. If the pH is below 9.7, the amine is in its protonated form, and has a positive charge. If the pH is above 9.7, the amine is in its normal form and has no charge. This is demonstrated by the green arrows on the pH scale below.
Now that we know which forms each functional group will have at different pH values, we can determine the overall form of alanine at a given pH. There are three forms of alanine below a cation, zwitterion, and anion. Each of these has different forms for its two functional group. For example, the alanine cation has the protonated amine and the carboxylic acid, while the zwitterion has the protonated amine but a carboxylate instead of the carboxylic acid. Let's assume we have a pH of 6. At this pH, the carboxylic acid would be in its carboxylate form and the amine is in its protonated amine form. Of the three forms below, only the zwitterion has both a carboxylate and a protonated amine, showing that at a pH of 6 the form of alanine is its zwitterion.
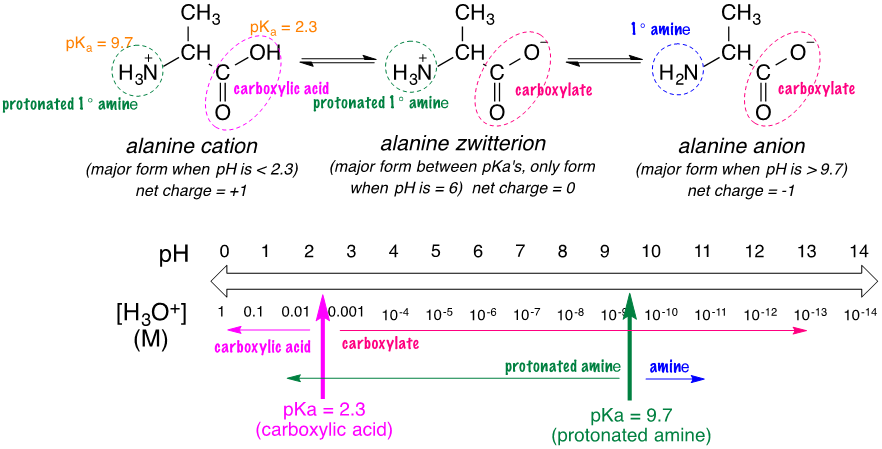
Figure \(\PageIndex{2}\): Protonation of Alanine

