Radical mechanisms for flavin-dependent reactions
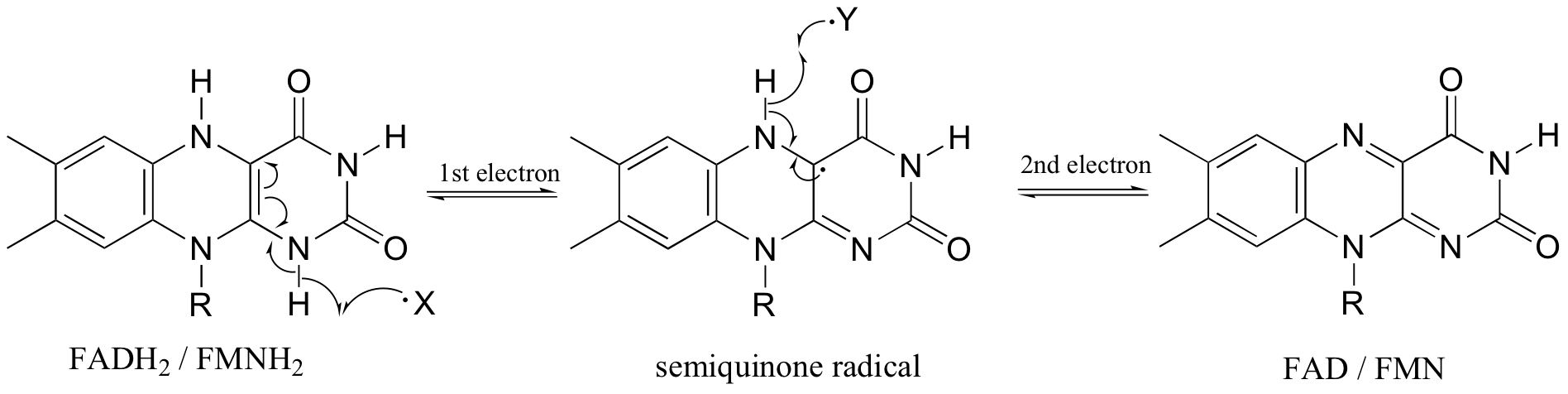
Flavin coenzymes, like their nicotinamide adenine dinucleotide counterparts, can act as hydride acceptors and donors. In these redox reactions, two electrons are transferred together in the form of a hydride ion. Flavin, however, is also capable of mediating chemical steps in which a single unpaired electron is transferred - in other words, radical chemistry. This is due to the ability of the flavin system to form a stabilized radical intermediate called a semiquinone, formed when FADH2 (or FMNH2) donates a single electron, or when FAD (or FMN) accepts a single electron.
This single-electron transfer capability of flavins is critical to their metabolic role as the entry point of electrons into the electron transport phase of respiration. Electrons 'harvested' from the oxidation of fuel molecules are channeled, one by one, by FMNH2 into the electron transport chain, where they eventually reduce molecular oxygen. NADH is incapable of single electron transfer - all it can do is transfer two electrons, in the form of a hydride, to FMN; the regenerated FMNH2 is then able to continue sending single electrons into the transport chain.
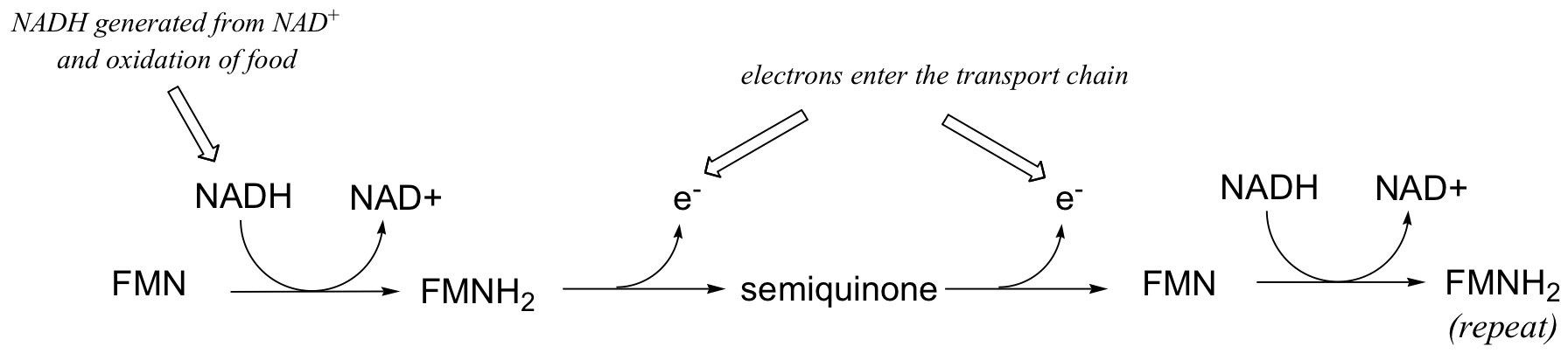
You will learn more details about this process in a biochemistry class.
Because flavins are capable of single-electron as well as two-electron chemistry, the relevant mechanisms of flavoenzyme-catalyzed reactions are often more difficult to determine. Recall the dehydrogenation reaction catalyzed by acyl-CoA dehydrogenase (section 16.5C) - it involves the transfer of two electrons and two protons (ie. a hydrogen molecule) to FAD. Both electrons could be transferred together, with the FAD coenzyme simply acting as a hydride acceptor (this is the mechanism we considered previously). However, because the oxidizing coenzyme being used is FAD rather than NAD+, it is also possible that the reaction could proceed by a single-electron, radical intermediate process. In the alternate radical mechanism proposed below, for example, the enolate intermediate first donates a single electron to FAD, forming a radical semiquinone intermediate (step 2). The second electron is transferred when the semiquinone intermediate abstracts a hydrogen from Cb in a homolytic fashion (step 3).
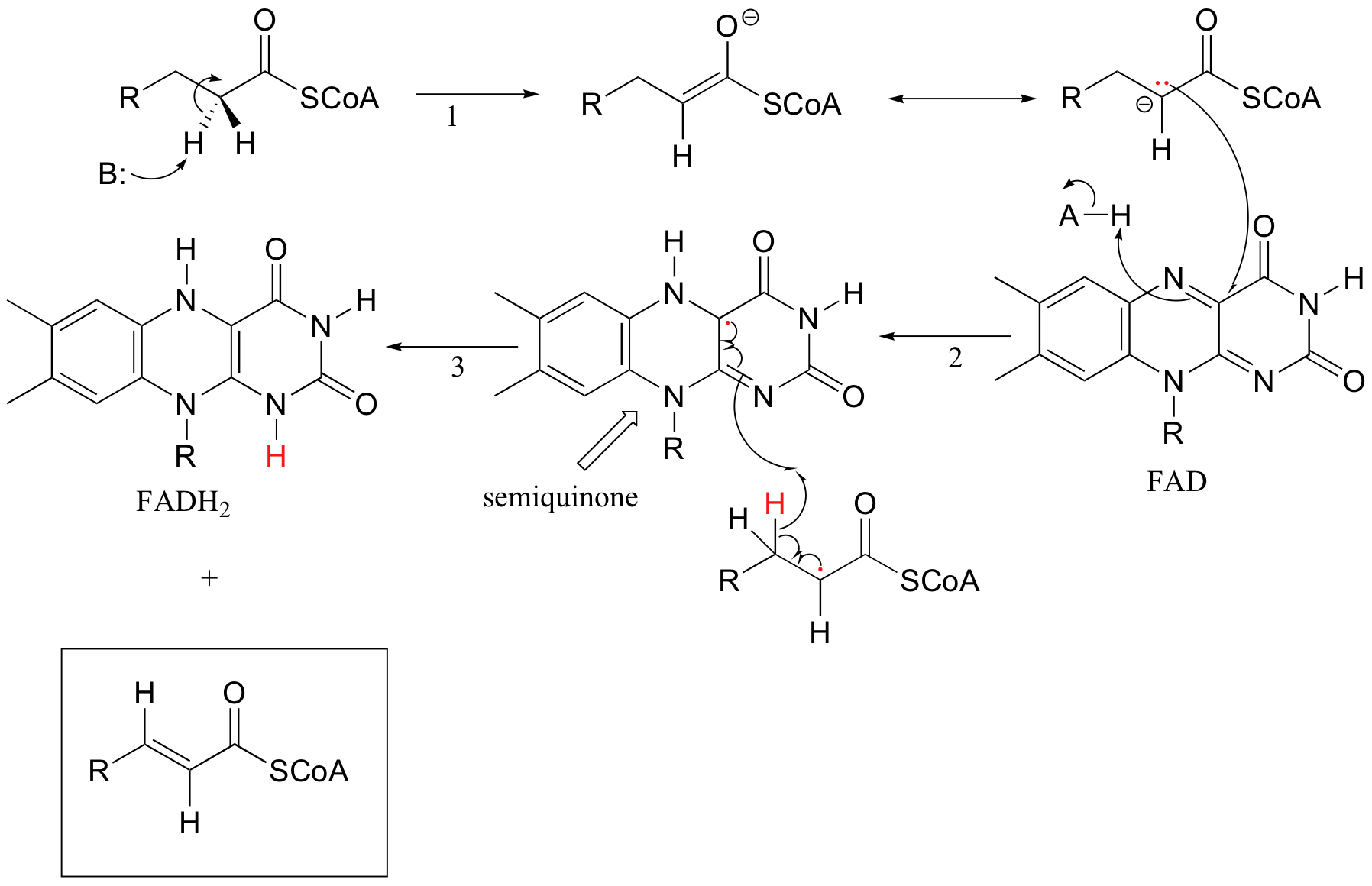
Scientists are still not sure which mechanism - the hydride transfer mechanism that we saw in section 16.5B or the single electron transfer detailed above - more accurately depicts what is going on in this reaction.
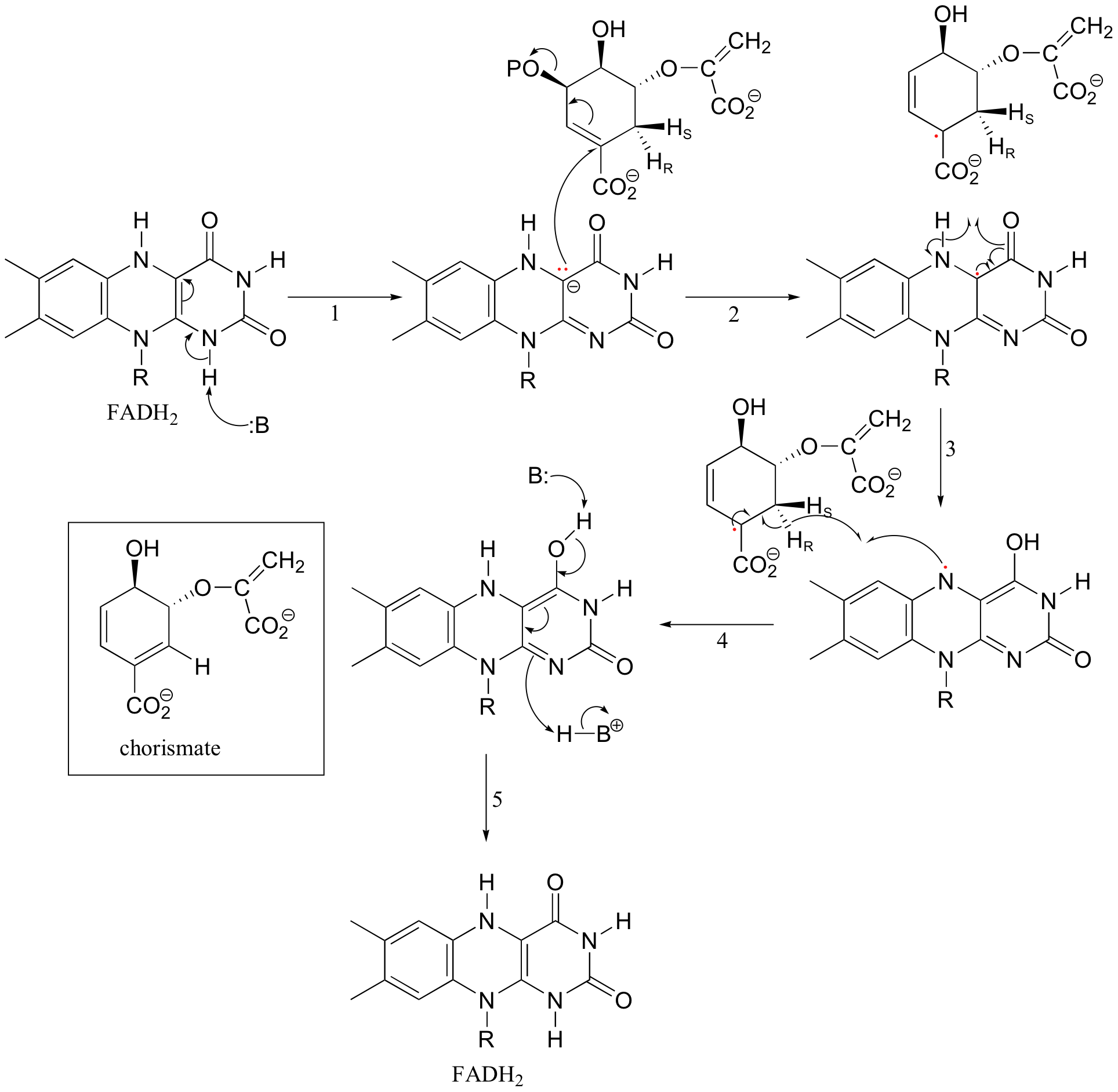
The conjugated elimination catalyzed by chorismate synthase (section 14.3B) is another example of a reaction where the participation of flavin throws doubt on the question of what is the relevant mechanism. This could simply be a conjugated E1' reaction, with formation of an allylic carbocation intermediate. The question plaguing researchers studying this enzyme, however, is why FADH2 is required. This is not a redox reaction, and correspondingly, FADH2 is not used up in the course of the transformation - it just needs to be bound in the active site in order for the reaction to proceed. Given that flavins generally participate in single-electron chemistry, this is an indication that radical intermediates may be involved. Recently an alternative mechanism, involving a flavin semiquinone intermediate, has been proposed (J. Biol. Chem 2004, 279, 9451). Notice that a single electron is transferred from substrate to coenzyme in step 2, then transferred back in step 4.